Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
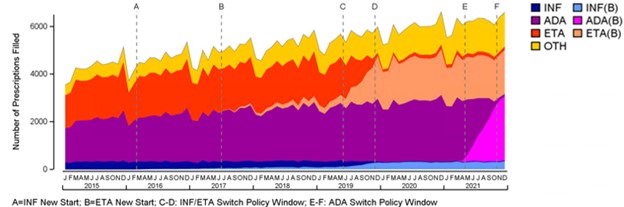
Background/Purpose: In response to the low uptake of biosimilars, British Columbia (BC) in Canada became the first jurisdiction in North America to require patients with inflammatory arthritis to switch to a biosimilar in order to maintain coverage. While the impact of this policy on etanercept (ETA) and infliximab (INF) has been previously reported, this study considered the impact of the policy on adalimumab (ADA) when the biosimilar became available in 2021.
Methods: We used administrative data from British Columbia (Population Data BC) to derive a cohort of patients with inflammatory arthritis being prescribed TNF inhibitor therapy before the policies were introduced. Previously established case definitions including ICD codes were used to establish patients with different inflammatory diseases. The data included both public and private coverage since commercial insurers providing supplementary drug coverage also aligned their policies with the BC Provincial government. Exceptional coverage for reference products was permitted under both biosimilar switching policies if medically necessary. We used descriptive statistics to analyze the trend of uptake pre and post the policy period, and a quasi-experimental interrupted time series analysis to consider the change in trends. We used interrupted time series analysis to estimate biosimilar uptake.
Results: The study identified 11,171 BC residents aged 18 years or older who were using a TNF inhibitor during the study period (01/2015-12/2021). The mean age of the cohort was 54 years and 59% were female. During the first switch policy that included mandatory switches for ETA and INF in 2019, biosimilar prescriptions increased from 7.9% to 35.0% of all TNF prescriptions. After the first switch period, there was a small but consistent decline in overall biosimilar use, with an increase in golimumab and certolizumab prescriptions. During the second switch policy in 2021 which focused on adalimumab, overall biosimilar prescriptions increased from 34% to 72% of all TNF inhibitor prescriptions. In December 2021, 96.4%, 93.0% and 92.0% of prescriptions for ADA, ETA and INF respectively were biosimilar products.
Conclusion: The study findings indicate that a mandatory switch policy for biosimilar adalimumab has been as successful as the policy for infliximab and etanercept achieving high biosimilar use in British Columbia. Prior to the policy, uptake was low indicating the need for such a policy to influence change. Further analysis will explore changes in other healthcare utilization and assess the long-term effects of these policies. These findings are particularly relevant to regions with concentrated insurance systems, as mandatory switching policies could yield greater cost savings.
To cite this abstract in AMA style:
Bansback N, law M, Clemont F, Tadrous M, Blitz S, Harrison M. How Did a Mandatory Switch Policy Influence the Uptake of Adalimumab Biosimilar and Other TNF Inhibitors? [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/how-did-a-mandatory-switch-policy-influence-the-uptake-of-adalimumab-biosimilar-and-other-tnf-inhibitors/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-did-a-mandatory-switch-policy-influence-the-uptake-of-adalimumab-biosimilar-and-other-tnf-inhibitors/