Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Some patients with antiphospholipid syndrome (APS) are afflicted by an insidious small-vessel vasculopathy that results in the accrual of organ damage over time. While the neotima formation that heralds this vasculopathy is most commonly visualized upon kidney biopsy, other organs such as the brain, heart, and skin may also be at risk. We previously used single-cell RNA sequencing of APS skin biopsies to identify the upregulation of various proliferation-supporting genes in microvascular endothelial cells (MVECs). The most notable example was CCN2 (also known as CTGF), a classic downstream target of Hippo-YAP1 signaling. Furthermore, the previous bioinformatic analysis demonstrated the potential for strong communication between secreted MVEC ligands such as CCN2 and vascular smooth muscle cells (VSMC) receptors—a potentially critical interaction given the important role of VSMC proliferation and migration in neointima formation. Here, we aimed to determine the potential role of the Hippo-YAP1-CCN2 axis in APS micro-vasculopathy.
Methods: Isolated healthy MVECs were cultured with APS patient serum or patient-derived IgG. CCN2 expression was measured by quantitative PCR and ELISA. Immunofluorescence (IF) microscopy and western blotting were used to assess YAP1 signaling. To study cellular communication, human VSMCs were cultured with conditioned media from APS-stimulated MVECs. The proliferation and migration of VSMCs were determined by BrdU-staining and wound-scratch assays, respectively. Finally, CCN2 expression was assessed in kidney biopsies from patients with APS nephropathy by IF microscopy.
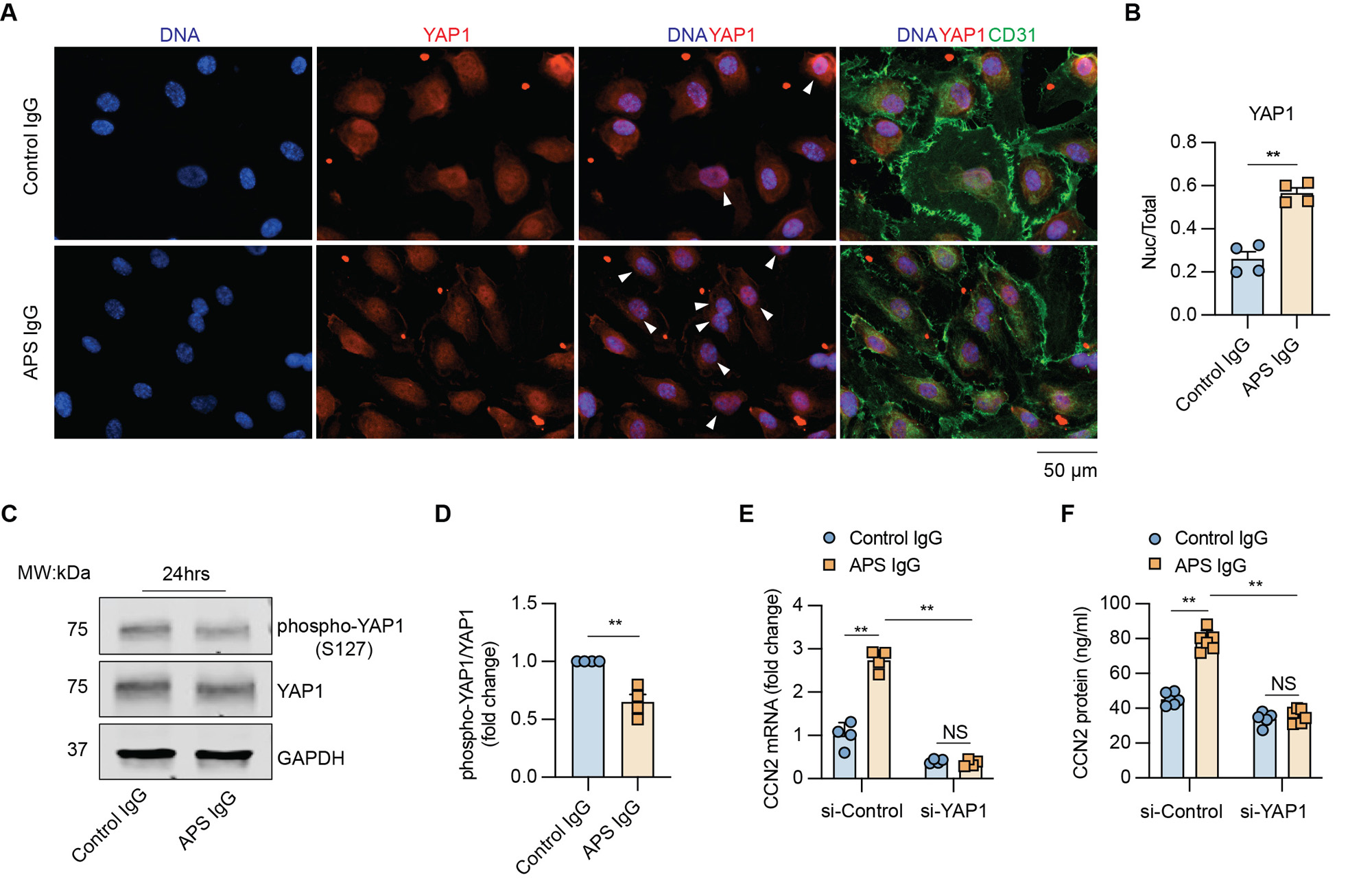
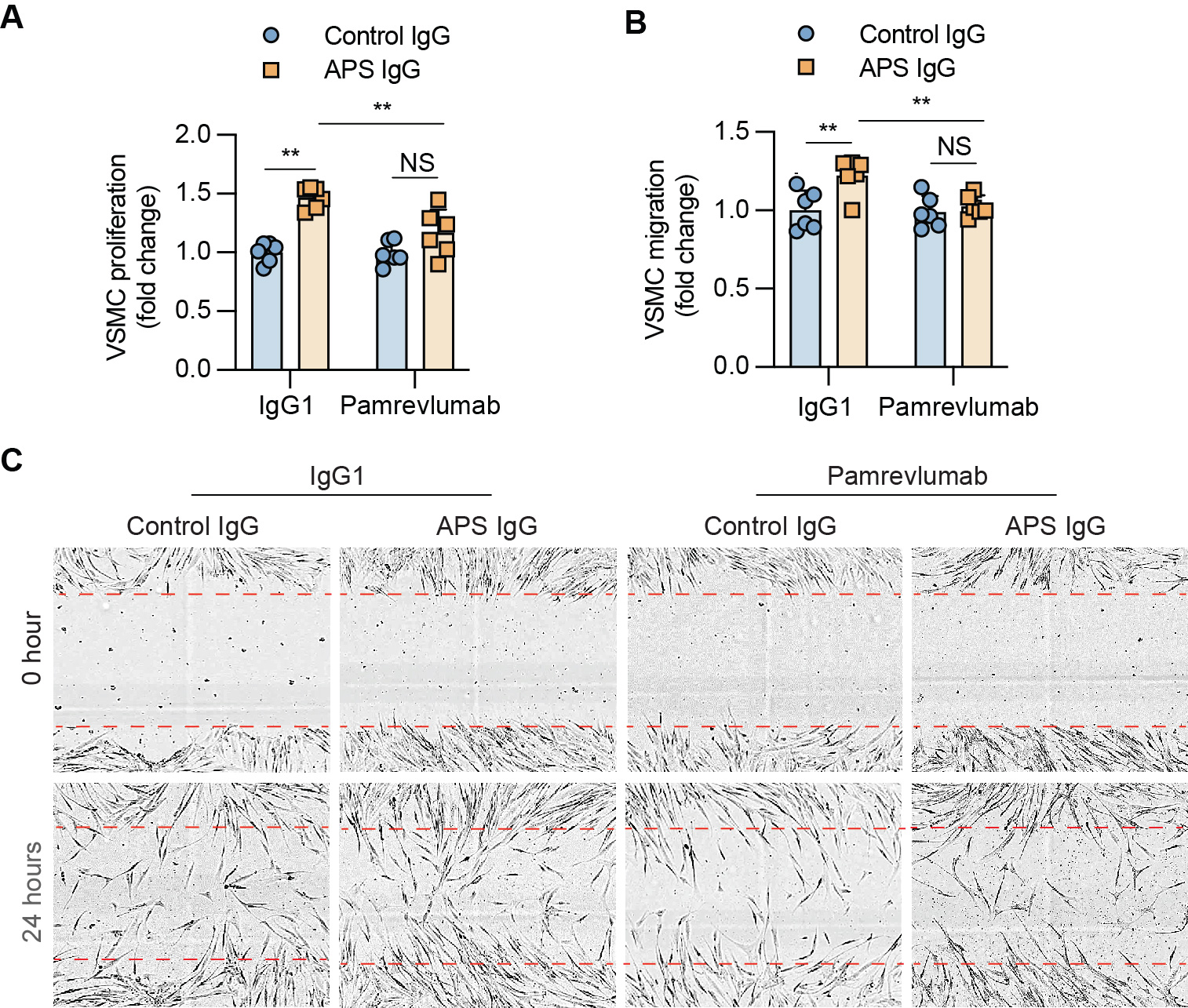
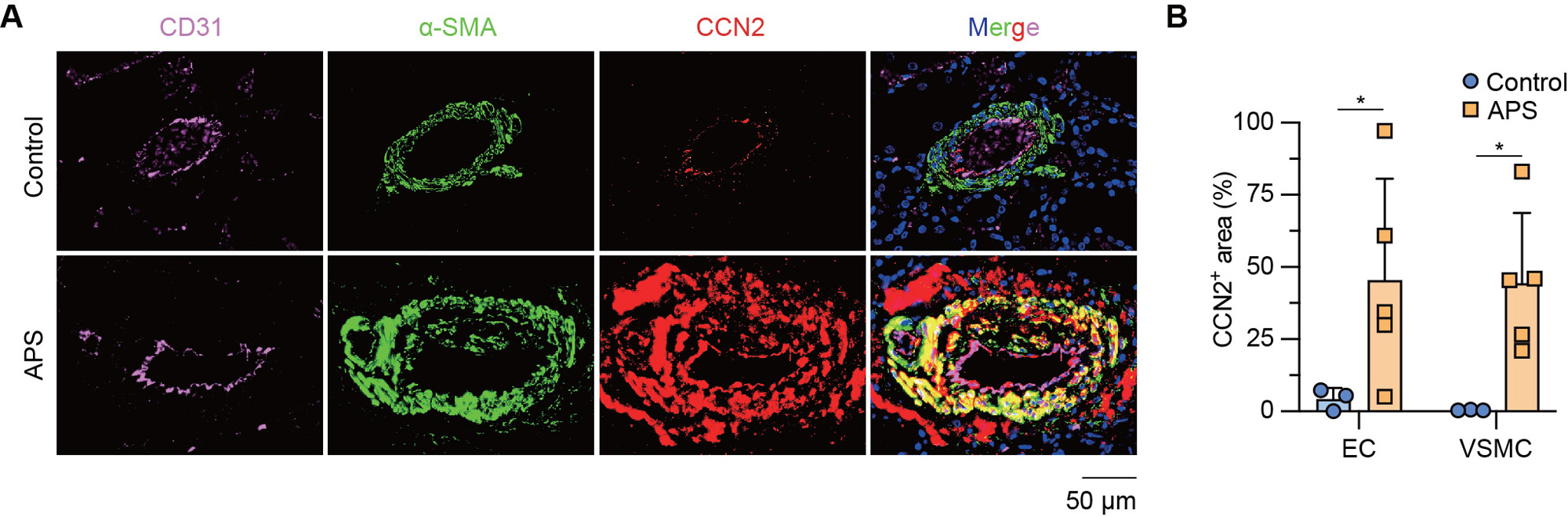
Results: Culture of MVECs with APS patient serum or patient-derived IgG led to upregulation of CCN2 mRNA (fold change 2.3 ± 0.3 and 2.8 ± 0.1, respectively, both p< 0.01). Concomitantly, secreted CCN2 protein in the supernatant was increased as assessed by ELISA (data not shown). In parallel studies, the activation of the Hippo-YAP1 pathway was confirmed by increased YAP1 nuclear translocation and reduced YAP1 phosphorylation (Figure 1A-D). Notably, the knockdown of YAP1 in MVECs abolished APS IgG-induced CCN2 expression and secretion (Figure 1E-F). Regarding MVEC-VSMC communication, conditioned media from APS IgG-stimulated MVECs triggered a noncontractile phenotype in VSMCs, accompanied by increased proliferation and migration. These phenotypes were mitigated by the humanized CCN2-blocking antibody, pamrevlumab (Figure 2). Finally, kidney biopsies from patients with APS nephropathy showed markedly enhanced CCN2 expression in the thickened intima and media of microvessels, indicating the in vivo relevance of this pathway (Figure 3).
Conclusion: We found that APS serum and patient-derived IgG triggered YAP1 nuclear translocation in MVECs, which was accompanied by increased CCN2 expression and secretion. The secreted CCN2 was then able to trigger a phenotypic switch in VSMCs toward the migratory and proliferative characteristics that are necessary for neointima formation. Blocking either YAP1 or CCN2 might be a novel approach for the treatment of APS-associated micro-vasculopathies.
To cite this abstract in AMA style:
Liang W, Billi A, Yalavarthi S, Rysenga C, Hoy C, Sarosh C, Zuo Y, Tsou E, Knight J, Shi H. Hippo-YAP1-CCN2 Signaling by Microvascular Endothelial Cells Licenses Vascular Smooth Muscle Cell Proliferation in Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/hippo-yap1-ccn2-signaling-by-microvascular-endothelial-cells-licenses-vascular-smooth-muscle-cell-proliferation-in-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hippo-yap1-ccn2-signaling-by-microvascular-endothelial-cells-licenses-vascular-smooth-muscle-cell-proliferation-in-antiphospholipid-syndrome/