Session Information
Date: Tuesday, November 14, 2023
Title: (1913–1944) Miscellaneous Rheumatic & Inflammatory Diseases Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Pediatric patients with autoinflammatory diseases (AID) on Canakinumab (CAN) therapy have been affected by the coronavirus pandemic including SARS-CoV-2 infection, SARS-CoV-2 vaccination, and AID disease management. In the RELIANCE registry, severity of SARS-CoV-2 infection, safety of SARS-CoV-2 vaccination and comparison of disease management before and during pandemic in pediatric patients with cryopyrin-associated periodic syndromes (CAPS), familial Mediterranean fever (FMF), hyper-IgD syndrome/mevalonate kinase deficiency (HIDS/MKD) and tumor necrosis factor receptor-associated periodic syndrome (TRAPS) on CAN therapy was investigated in clinical practice.
Methods: The RELIANCE registry is a prospective, non-interventional, observational study in Germany enrolling pediatric (age ≥2 years) and adult patients with a clinically confirmed diagnosis of AID who routinely receive CAN. Efficacy and safety parameters are recorded at baseline and assessed at 6-month intervals.
Results: The present interim analysis includes data from n=101 pediatric patients with AID enrolled in the RELIANCE registry between October 2017 and December 2022. The median duration of CAN treatment before and during the study was 3.7 years (0-13.5 years).
During the study, 29 SARS-CoV-2 infections were reported for n=27 pediatric patients. 22 infections caused mild symptoms and 7 infections caused moderate symptoms. N=25 (24.8 %) of pediatric patients received at least one SARS-CoV-2 vaccination (15 Comirnaty, 13 not specified). Vaccination reactions (not further specified) were reported in n=3 patients. No reactions were classified as serious.
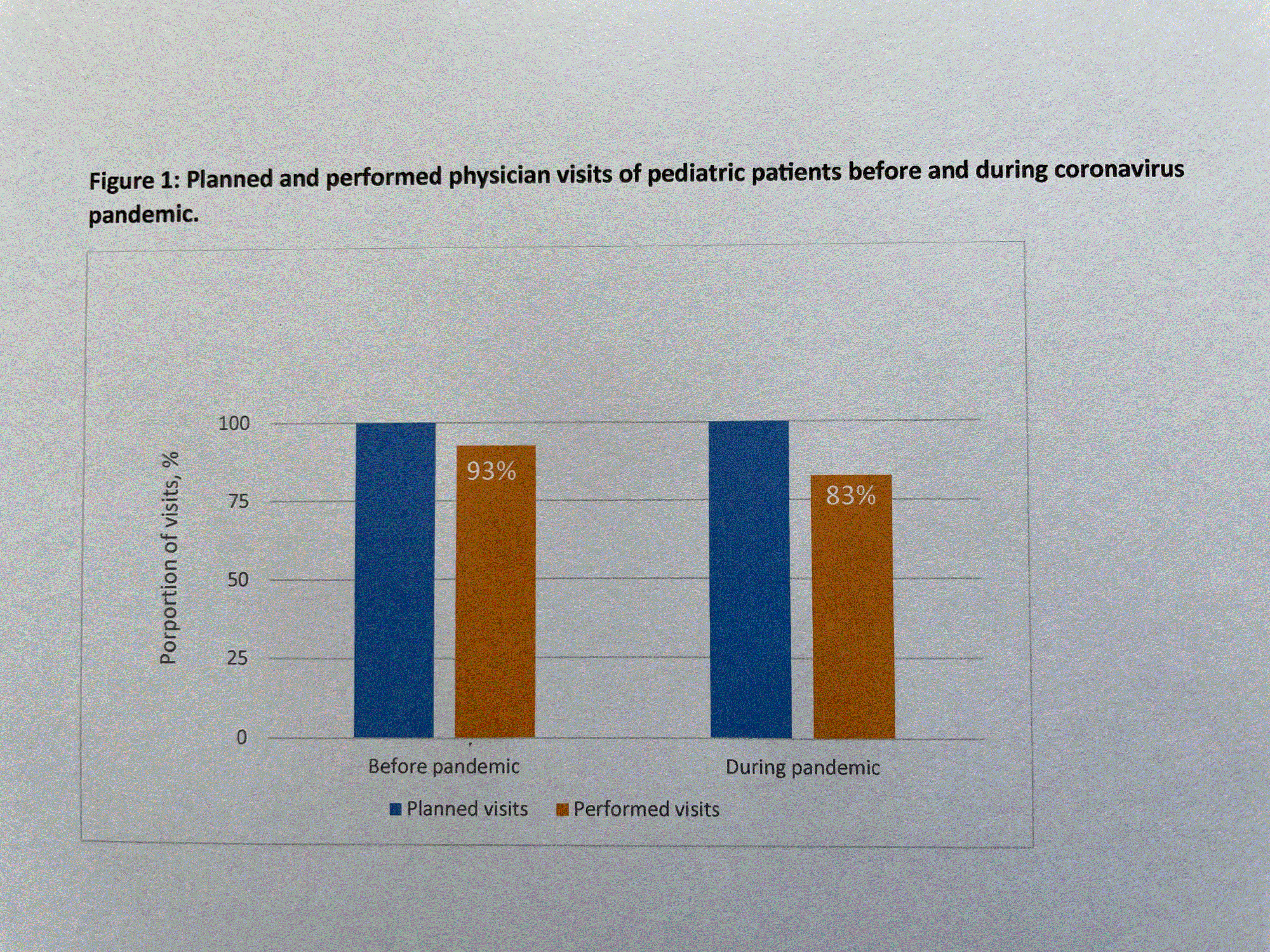
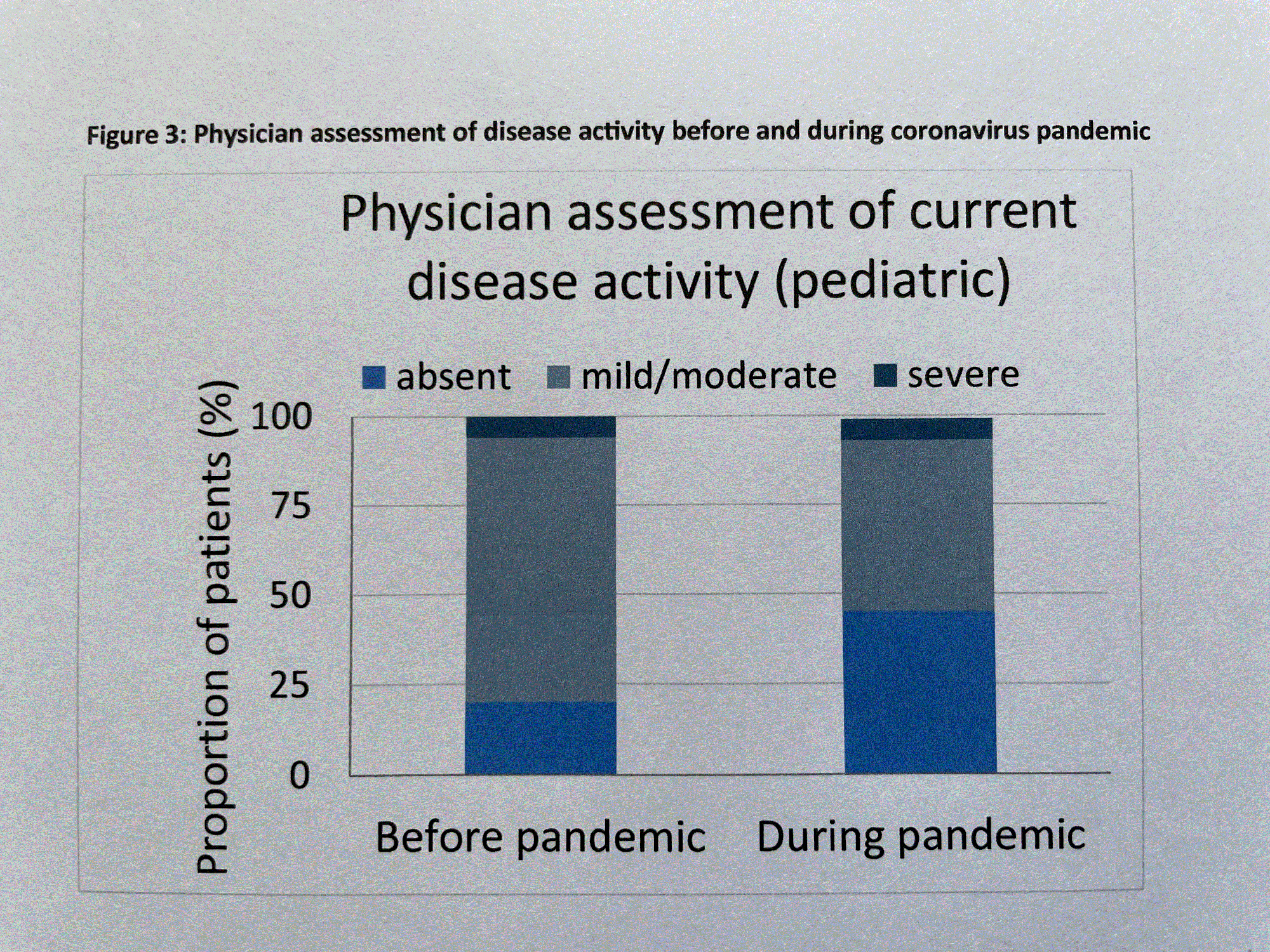
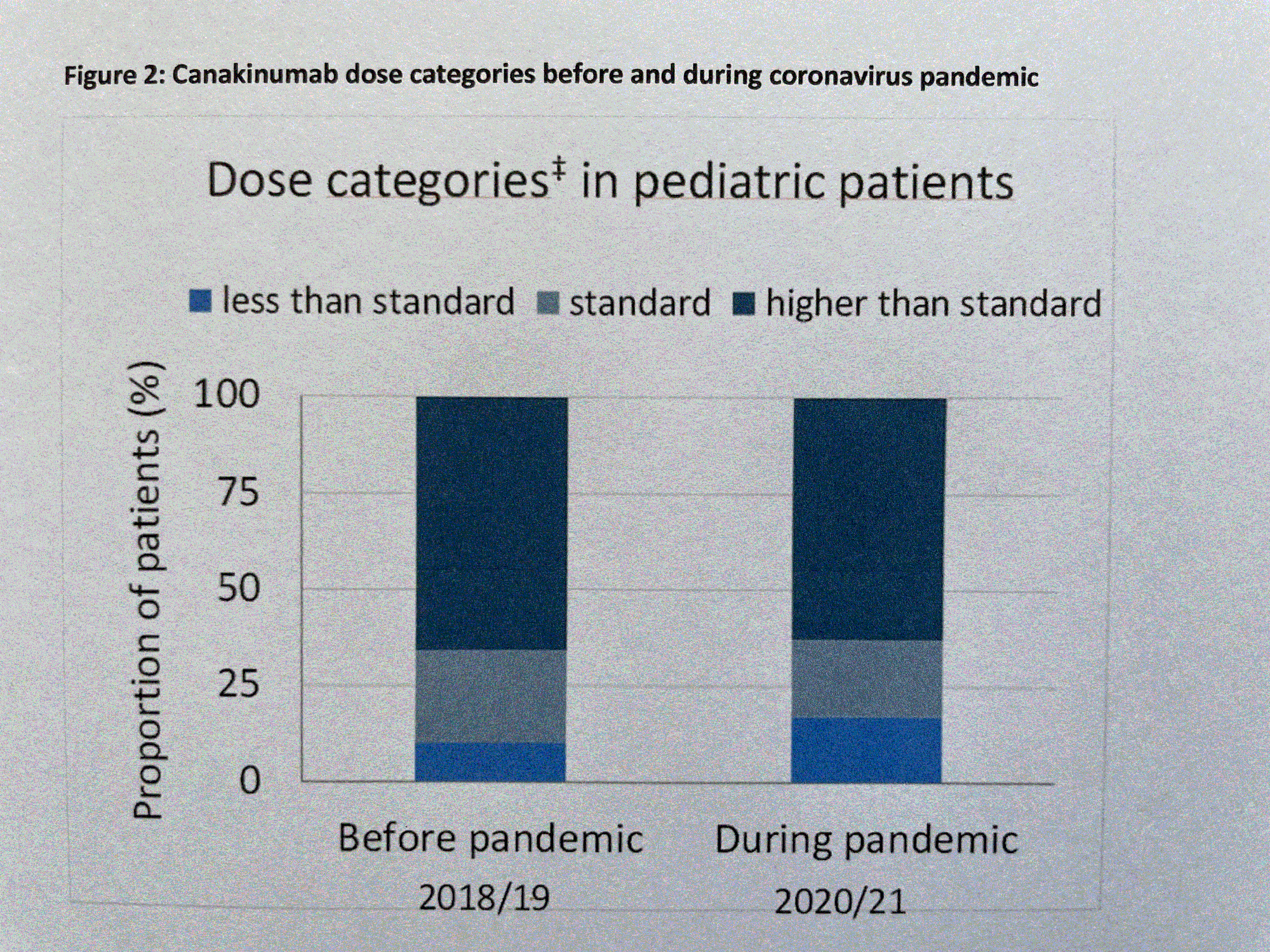
During the COVID-19 pandemic in 2020 and 2021, only 83 % of regular patient visits were performed (Fig. 1). In addition, patients received slightly lower CAN dosing (20% standard dose CAN [SD], 17% lower than SD, and 63% higher than SD compared to 24% SD, 10% lower than SD, and 66% higher than SD before pandemic (Fig. 2). However, disease activity by patient rating (VAS score 1-10) improved from 3 before to 2 during the pandemic. By physician global assessment, the proportion of pediatric patients with no disease activity arose from 20% before to 45% during the pandemic (Fig. 3). In contrast: no major changes in disease activity were documented in adult patients.
Conclusion: Pediatric AID patients under long-term Canakinumab treatment experienced improved disease control during the Coronavirus pandemic.
To cite this abstract in AMA style:
Horneff G, Blank N, Kuemmerle-Deschner J, Henes J, Kortus-Goetze B, Oommen P, Pankow A, Krickau T, Schuetz C, Foeldvari I, Rech J, Weller-Heinemann F, Janda A, Hufnagel M, Meier F, Dressler F, Borte M, Andreica I, Wasiliew P, Fiene M, Windschall D, Krusche M, Kuempfel T, Weber-Arden J, Kallinich T. Higher Rates of Disease Control During the Coronavirus Pandemic in Pediatric Patients with Autoinflammatory Periodic Diseases on Canakinumab Treatment – Interim Data from the RELIANCE Registry [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/higher-rates-of-disease-control-during-the-coronavirus-pandemic-in-pediatric-patients-with-autoinflammatory-periodic-diseases-on-canakinumab-treatment-interim-data-from-the-reliance-registry/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/higher-rates-of-disease-control-during-the-coronavirus-pandemic-in-pediatric-patients-with-autoinflammatory-periodic-diseases-on-canakinumab-treatment-interim-data-from-the-reliance-registry/