Session Information
Date: Saturday, November 6, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster I: Impact (0225–0240)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The MATURE study investigated administration of 300 mg secukinumab (SEC) using a 2 mL autoinjector (AI) device. The objective of this study was to assess efficacy, safety, tolerability, and pharmacokinetics (PK) of the 2 mL AI for SEC 300 mg in patients with moderate to severe plaque psoriasis (PsO).
Methods: MATURE was a multicentre, randomised, double-blind, placebo (PBO)-controlled, parallel-group Phase III trial (NCT03589885) conducted at 22 sites worldwide. The study consisted of 3 periods: screening (screening to baseline [BL]), treatment period 1 (BL to Week 12; pre-dose), and treatment period 2 (Week 12 dose to Week 52). Eligible patients were randomised to receive SEC 300 mg in a 2 mL AI or 2x 1 mL pre-filled syringe (PFS) or PBO. The co-primary endpoints were Psoriasis Area and Severity Index (PASI) 75 and Investigator’s Global Assessment (IGA) modified 2011 0/1 responses at Week 12. The key secondary endpoint was PASI 90 response at Week 12. Other secondary endpoints were PK assessments, PASI 75/90/100 responses, DLQI score of 0/1, usability of 2 mL AI (rated through a Self-Injection Assessment Questionnaire [SIAQ]), and safety over a period of 52 weeks.
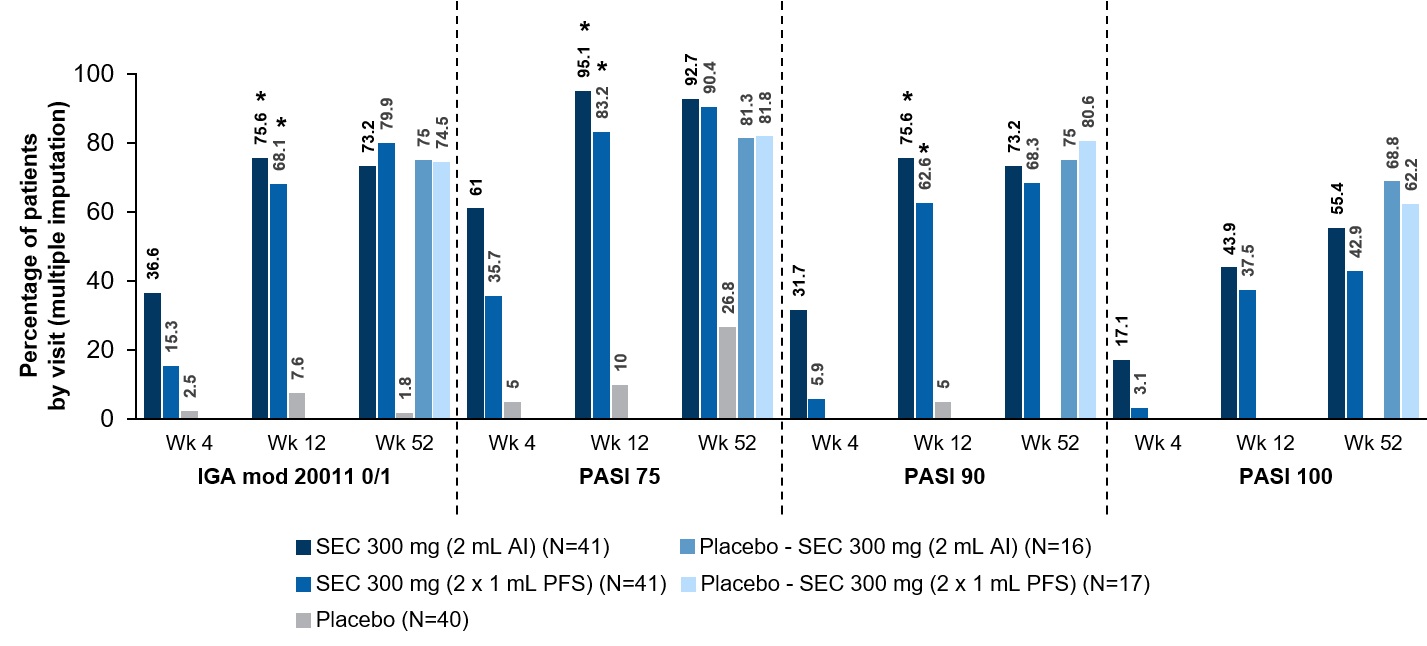
Results: In total, 122 patients were randomised as follows: SEC 300 mg 2 mL AI (N=41), SEC 300 mg 2x 1 mL PFS (N=41), or PBO (N=40). The study met both co-primary and key secondary endpoints (p< 0.0001). At Week 12, SEC 300 mg 2 mL AI and 2x 1 mL PFS showed superior PASI 75 response rates (95.1% and 83.2%, respectively), and IGA mod 2011 0/1 response rates (75.6% and 68.1%, respectively), compared with PBO (10.0% for PASI 75; 7.6% for IGA mod 2011 0/1) (Fig. 1). At Week 12, the PASI 90 response was significantly higher with both SEC groups (2 mL AI [75.6%] and 2x 1 mL PFS [62.6%]) than with PBO (5.0%; p< 0.0001 for both) (Fig. 1). Both SEC treatment groups (2 mL AI and 2x 1 mL PFS) showed a similar trend of efficacy to Week 52 (Fig. 1). Similarly, higher DLQI 0/1 response rates were observed in both SEC groups (2 mL AI [71.1%] and 2x 1 mL PFS [72.5%] at Week 12) compared with PBO (8.1%); the trend of high DLQI 0/1 response was sustained up to Week 52 in both SEC groups. The higher mean SEC concentration observed with the 2 ml AI, may explain the slightly faster and numerically higher PASI 90 response. This difference did not affect the incidence of adverse events in either group. SIAQ results showed high usability of self-injection with the 2 mL AI device. In the secukinumab 300 mg (2 mL AI) group, the proportion of “very satisfied” and “satisfied” patients increased substantially from 31.6% before the injection (pre-SIAQ at baseline) to 78.3% (post-SIAQ at baseline) after the first injection and remained above 85% at later visits, reaching 100% at Week 28. Throughout the entire treatment period, only two patients (1.7%) reported one “injection-site reaction” each; both events were attributed to the AI administration (one of them with active drug, the other one with placebo). No new safety signals were observed with the use of the AI over the 52-week period.
Conclusion: SEC 300 mg administered with the 2 mL AI demonstrated superior efficacy over PBO, good tolerability, and convenience of administration in patients with moderate to severe plaque PsO.
To cite this abstract in AMA style:
Sigurgeirsson B, Browning J, Tyring S, Szepietowski J, Rivera‐Díaz R, Effendy I, Keefe D, Bruin G, Paguet B, Fu R, Hampele I, Reinhardt M. High Efficacy, Safety, and Tolerability of Secukinumab Injection with 2 mL Auto-injector (300 Mg) in Adult Patients with Moderate to Severe Plaque Psoriasis: 52-week Results from MATURE, a Randomised, Placebo-controlled Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/high-efficacy-safety-and-tolerability-of-secukinumab-injection-with-2-ml-auto-injector-300-mg-in-adult-patients-with-moderate-to-severe-plaque-psoriasis-52-week-results-from-mature-a-randomised/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-efficacy-safety-and-tolerability-of-secukinumab-injection-with-2-ml-auto-injector-300-mg-in-adult-patients-with-moderate-to-severe-plaque-psoriasis-52-week-results-from-mature-a-randomised/