Session Information
Date: Sunday, November 10, 2019
Title: 3S076: Patient Outcomes, Preferences, & Attitudes I: Patient Reported Outcomes (833–838)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
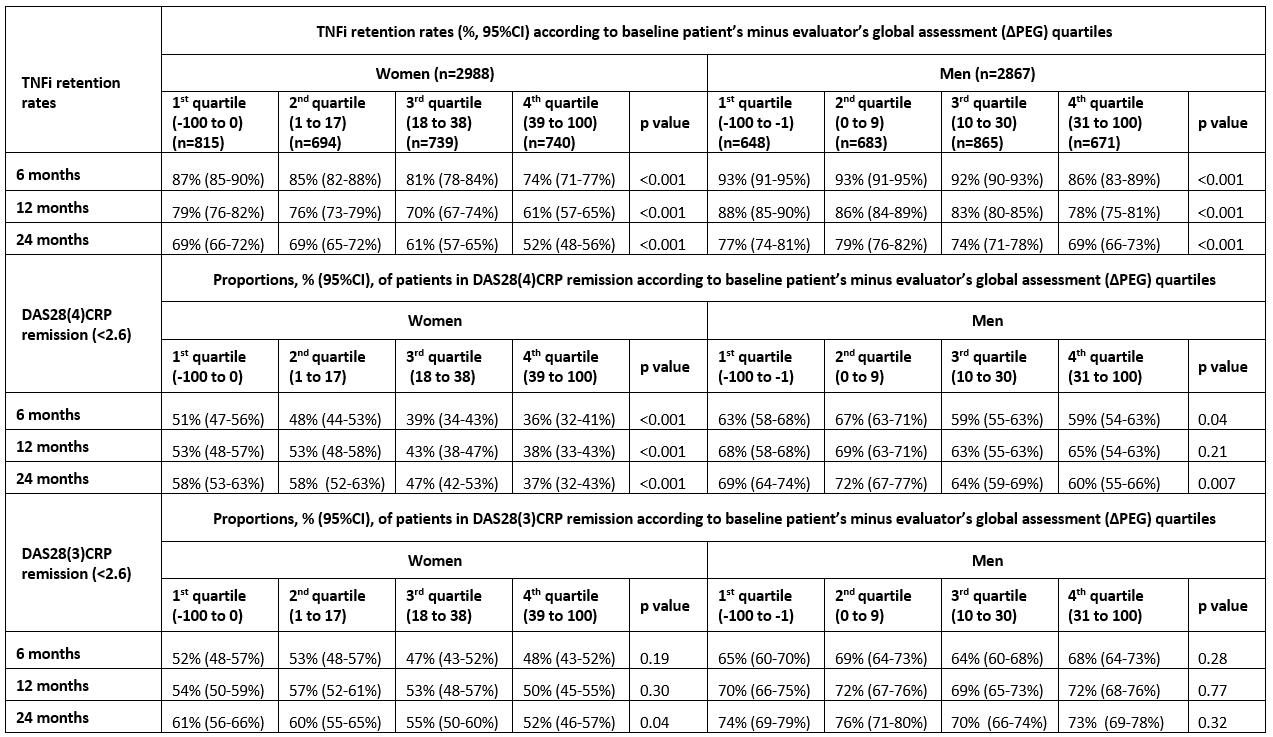
Background/Purpose: Discordance between baseline patient’s and evaluator’s global assessment of disease activity is common1 and may reduce the likelihood of remission following tumor necrosis factor inhibitor (TNFi) treatment in patients with psoriatic arthritis (PsA).2 However, the impact of such discordance on retention rates of TNFi in PsA patients remains unexplored. Hence, the aim of this study was to explore the impact of discordance, defined as patient’s minus evaluator’s global assessment (ΔPEG), on retention rates and remission rates (DAS28(3)CRP (without patient’s global) and DAS28(4)CRP (including patient’s global)) in PsA patients initiating their first TNFi treatment. We used pooled data from the European Spondyloarthritis Research Collaboration Network (EuroSpA).
Methods: TNFi naïve PsA patients from 11 European registries in EuroSpA were included. Kaplan-Meier analyses were used to estimate TNFi retention rates after 6/12/24 months, with comparison between baseline ΔPEG quartiles using the log rank test, stratified by gender. Remission rates were compared between different ΔPEG quartiles with Chi-square test, stratified by gender.
Results: A total of 5855 PsA patients were included. Mean (SD) age for women (n=2988) / men (n=2867) were 49.3 (12.5) / 47.4 (11.7) years, disease duration 6.6 (7.3) / 6.7 (7.2) years, median (25-75 percentiles) baseline ΔPEG 17 (0-38) / 10 (0-30) mm. Retention rates and DAS28(4)CRP but not DAS28(3)CRP remission rates were lower for higher quartiles of baseline ΔPEG (table, figure).
Conclusion: High baseline discordance (ΔPEG) was associated with lower TNFi retention rates and with lower DAS28(4)CRP remission rates, but not DAS28(3)CRP remission rates, after 6, 12 and 24 months’ follow-up in both male and female PsA patients. The choice of remission criteria in the follow-up of PsA patients may affect important treatment decisions, and may be of particular impact in patients with high baseline ΔPEG.
To cite this abstract in AMA style:
Michelsen B, Midtbøll Ørnbjerg L, Mann H, Kvien T, Nissen M, Santos M, Nordström D, Jacobsson L, Rotar Z, Gudbjornsson B, Koca S, Codreanu C, Pombo-Suarez M, van der Horst-Bruinsma I, Loft A, Pavelka K, Kristianslund E, Moeller B, Vieira-Sousa E, Hokkanen A, Lindström U, Tomsic M, Love T, Tufan A, IONESCU R, Sánchez-Piedra C, van de Sande M, Macfarlane G, Iannone F, Hyldstrup L, Østergaard M, Lund Hetland M. High Baseline Patient’s Compared with Evaluator’s Global Assessment Is Associated with Lower Retention and Remission Rates of First TNF Inhibitor in Psoriatic Arthritis Patients – Data from the EuroSpA Research Collaboration Network [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/high-baseline-patients-compared-with-evaluators-global-assessment-is-associated-with-lower-retention-and-remission-rates-of-first-tnf-inhibitor-in-psoriatic-arthritis-patients-data/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-baseline-patients-compared-with-evaluators-global-assessment-is-associated-with-lower-retention-and-remission-rates-of-first-tnf-inhibitor-in-psoriatic-arthritis-patients-data/