Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In the phase 2 MUSE and phase 3 TULIP-1 and TULIP-2 trials, treatment with anifrolumab, a monoclonal antibody to the type I interferon receptor, was associated with clinical benefit in patients (pts) with SLE.1–3 In all 3 trials, herpes zoster (HZ) was observed more frequently with anifrolumab vs placebo. We conducted an integrated analysis to characterize the frequency and nature of HZ events with anifrolumab across the MUSE and TULIP trials.
Methods: MUSE, TULIP-1, and TULIP-2 were randomized, double-blind, 52-wk trials that evaluated anifrolumab 300 mg (IV Q4W for 48 wks) or placebo in pts with moderately to severely active SLE despite SOC.1–3 MUSE also included a 1000-mg treatment arm, and TULIP-1 included a 150-mg treatment arm; these pts were analyzed separately. We assessed HZ AE incidence, severity, intensity, discontinuation, and time to onset.
Results: In pooled data from MUSE, TULIP-1, and TULIP-2, 459 pts received ≥1 dose of anifrolumab 300 mg and 466 received ≥1 dose of placebo. In addition, 93 pts received anifrolumab 150 mg in TULIP-1 and 105 received 1000 mg in MUSE. HZ occurred in 5.4% (n=5), 6.1% (n=28), and 8.6% (n=9) of pts in the anifrolumab 150-mg, 300-mg, and 1000-mg groups, respectively, and in 1.3% (n=6) of the pooled placebo group (Table 1). Forty-four pts had HZ AEs that were mild or moderate, and 4 had events that were severe (anifrolumab 300 mg, n=2; 1000 mg, n=2). Three pts had serious AEs of HZ (anifrolumab 300 mg, n=2; 1000 mg, n=1). Of the 48 pts with HZ AEs, all but 4 continued in the study and all AEs leading to discontinuation were nonserious and moderate (anifrolumab 150 mg, n=1; 300 mg, n=2; 1000 mg, n=1). In pooled data available from TULIP-1 and -2, all HZ AEs with anifrolumab 300 mg were cutaneous (21 localized, unilateral; 2 disseminated, unilateral); all 5 HZ AEs with placebo were localized cutaneous. HZ resolved in all cases in MUSE and TULIP; all pts in the anifrolumab group and 4 of 6 pts in the placebo group received antiviral treatment. Using pooled TULIP data, no pattern of HZ event frequency was identified in protocol-defined subgroups. Pts using immunosuppressants (IS; n=349) had a difference in the rate of HZ between anifrolumab vs placebo groups of 8.1% (CI 2.6, 13.7) and those without IS use (n=376) had a difference of 2.2% (CI –1.8, 6.2) (Figure 1). Time to first onset of HZ was marginally shorter in the anifrolumab 300-mg vs placebo group (Figure 2).
Conclusion: In MUSE and TULIP trials, there appears to be an increased risk of HZ with anifrolumab vs placebo. HZ event characteristics, including duration and severity, were comparable between treatment groups, and most HZ events were mild to moderate, cutaneous, and resolved without discontinuation of study drug.
References
- Furie R. Arthritis Rheumatol. 2017;69:376–86.
- Furie R. Lancet Rheumatol. 2019;1:e208–19.
- Morand EF. N Engl J Med. 2020;382:211–21.
Writing assistance by Angela Cimmino, PharmD (JK Associates Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
 Table 1. Herpes Zoster Events During Treatment With Anifrolumab 300 mg vs Placebo in Pooled MUSE, TULIP-1, and TULIP-2 Data, 150 mg vs Placebo in TULIP-1, and 1000 mg vs Placebo in MUSE
Table 1. Herpes Zoster Events During Treatment With Anifrolumab 300 mg vs Placebo in Pooled MUSE, TULIP-1, and TULIP-2 Data, 150 mg vs Placebo in TULIP-1, and 1000 mg vs Placebo in MUSE
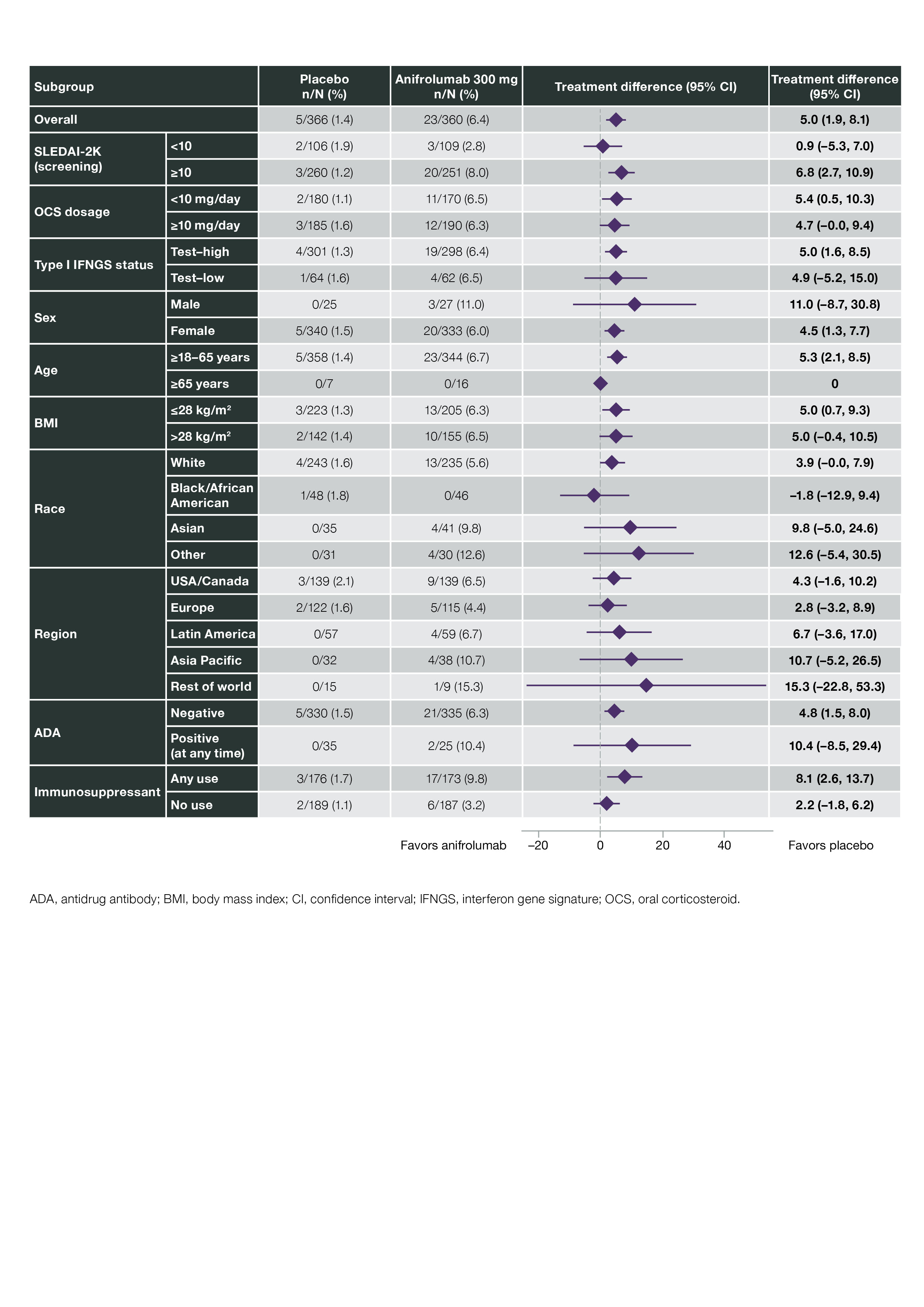 Figure 1. Adjusted Difference in Cumulative Proportions of Patients With Herpes Zoster Events With Anifrolumab 300 mg vs Placebo in Pooled TULIP-1 and TULIP-2 Data
Figure 1. Adjusted Difference in Cumulative Proportions of Patients With Herpes Zoster Events With Anifrolumab 300 mg vs Placebo in Pooled TULIP-1 and TULIP-2 Data
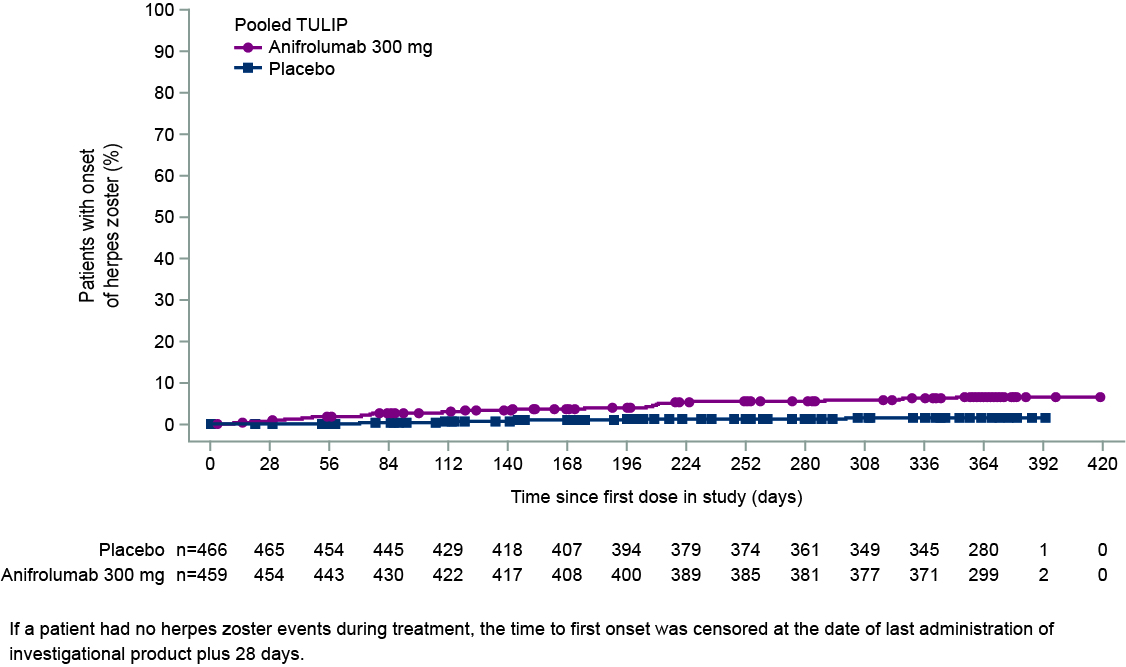 Figure 2. Time to First Onset of Herpes Zoster During Treatment With Anifrolumab 300 mg vs Placebo in Pooled MUSE, TULIP-1, and TULIP-2 Data
Figure 2. Time to First Onset of Herpes Zoster During Treatment With Anifrolumab 300 mg vs Placebo in Pooled MUSE, TULIP-1, and TULIP-2 Data
To cite this abstract in AMA style:
Merrill J, Kalunian K, Furie R, Winthrop K, Primakov P, Pineda L, Abreu G, Tummala R. Herpes Zoster Events with Anifrolumab in Patients with Active SLE: An Integrated Analysis of Phase 2 and Phase 3 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/herpes-zoster-events-with-anifrolumab-in-patients-with-active-sle-an-integrated-analysis-of-phase-2-and-phase-3-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/herpes-zoster-events-with-anifrolumab-in-patients-with-active-sle-an-integrated-analysis-of-phase-2-and-phase-3-trials/
