Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Systemic Lupus Erythematosus (SLE) has a significant impact on patient’s quality of life. Fatigue is the most common symptom of SLE and affects between 50-80% of SLE patients (Cleanthous et al. 2012; Tench et al. 2000; Krupp et al. 1990). Our objective was to evaluate the health related quality of life burden in SLE in a clinical trial and to explore the relationship between fatigue and overall health status.
Methods:
Pooled treatment group data from a Phase Ib dose-escalation clinical trial study of adult patients with moderate to severe SLE were analyzed. Clinical trial outcomes included clinician-reported global assessment of disease severity (MGDA) and PRO measures: Short Form 36-item Health Survey, Version 2 (SF-36v2), Fatigue Severity Scale (FSS), and patient numeric rating scale (NRS) global assessment. Descriptive analyses were conducted to characterize overall burden of SLE compared to US general population. The relationship between fatigue and overall health status was evaluated by comparing SF-36v2, patient and physician global assessments changes at endpoint from baseline between fatigue responders and non-responders. A fatigue response was defined as FSS change score ≤-1.0.
Results:
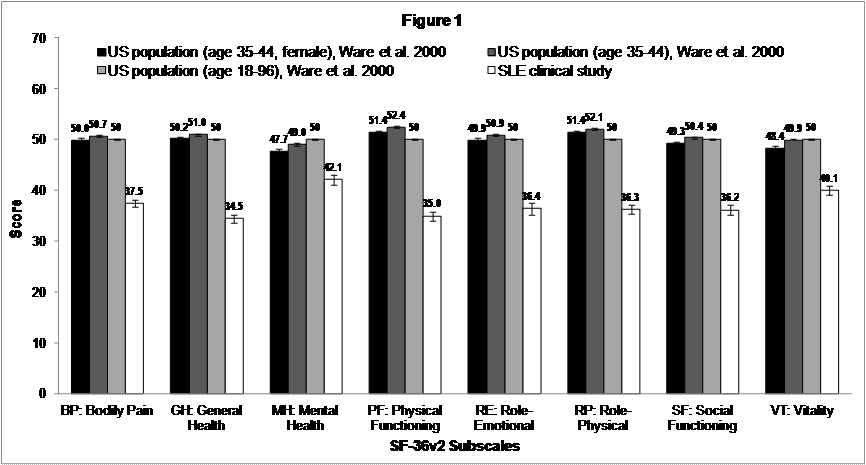
There were 161 patients, predominantly female (96%) and white (72%), with average age of 43±11 years (range: 18-71). Mean SF-36v2 subscale scores ranged from 34.5 (SD=9.6) for general health (GH) to 42.1 (SD=13.2) for mental health (MH); summary component scores reflected overall problems with physical (PCS; mean=35.2, SD=9.7) and mental health (MCS; mean=40.9, SD=12.9). SLE patients had worse health status on all SF-36v2 subscale domains than US general population and comparable age and gender norms (effect size [ES] = -0.51 to -2.15; Figure 1). A comparison of change scores between fatigue responders and non-responders showed that fatigue responders had greater improvement on SF-36v2 and patient and physician global assessments than non-responders. Fatigue responders had larger ES improvements than non-responders on SF-36v2 bodily pain (BP; ES=0.6 vs. 0.2), physical functioning (PF; ES=0.6 vs. 0.1), social functioning (SF; ES=0.6 vs. 0.0), and PCS (ES=0.7 vs. 0.1), patient global assessment NRS (ES=-0.7 vs. 0.0), and MDGA (ES=-1.6 vs. -0.7).
Conclusion:
SLE patients had poor HRQL in this study. All SF-36 domains including physical and mental health components were worse than general population averages. Improvement in fatigue was associated with improvements in the individual SF-36 domains as well as the physician’s global assessment of disease activity.
Figure 1: Comparison of Mean Baseline SF-36v2 Subscale Scores in the SLE Clinical Study to US Population Norms
Disclosure:
M. Petri,
MedImmune LLC, UCB, Pfizer, HGS, GSK, TEVA, Anthera,
9;
A. K. Kawata,
MedImmune LLC,
5;
A. W. Fernandes,
MedImmune LLC,
3;
K. Gajria,
MedImmune LLC,
3;
W. Greth,
MedImmune LLC,
3;
A. Hareendran,
MedImmune LLC,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-status-burden-and-impact-of-fatigue-on-patient-functioning-in-sle-patients-from-a-phase-1b-study/