Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background. Sjogren’s Syndrome (SS) is a chronic, autoimmune disease affecting the exocrine glands that may negatively affect health-related quality of life (HRQL). The importance of measuring symptoms and impacts has increasingly been recognized in patient care and clinical trials. Patient Reported Outcome Measurement Information System (PROMIS) provides universal HRQL instruments, but has not been previously implemented in SS. Currently, EULAR Sjogren’s Syndrome Patient Reported Index (ESSPRI) is widely utilized in SS but is limited in measuring HRQL domains. We sought to evaluate how selected PROMIS domains in SS would compare to US normative values and how scores may correlate with the ESSPRI.
Methods: Methods. This was a cross-sectional evaluation of adult patients with SS who completed PROMIS short-forms (SF) (depression 4a, anxiety 4a, fatigue 8a, physical function 10a, pain interference 8a, sleep disturbance 4a, participation in social roles and activities 8a) and had a clinical assessment between May 2018-March 2019. PROMIS T-scores are normalized to a score of 50 with a standard deviation of 10 in the US population; higher scores indicate more of the concept being measured; and a clinically meaningful difference is generally considered ½ SD, or 5 T-score units for some domains. Descriptive statistics, including means and proportions, were calculated for disease-related and sociodemographic variables. Pearson correlation was used to evaluate the relationship between subdomains of the ESSPRI and PROMIS.
Results: Results. Ninety-nine individuals met the 2016 ACR criteria for SS and completed PROMIS SF and clinical measures. Individuals with SS had a median age (IQR) of 57 (43, 66) and were mostly female (90%) and white (81%) (Table 1). Median (IQR) ESSPRI scores were 5.33 (3.66, 7), indicating moderate patient-reported disease activity; median (IQR) ESSRPI subdomain scores were 5 (2, 7) for pain, 6 (5, 8) for dryness, and 6 (4, 7) for fatigue. PROMIS scores for pain interference, fatigue, physical function, and anxiety were at least ½ SD worse than US population normative values. PROMIS pain interference (r=0.78) and fatigue (r=0.79) highly correlated with ESSPRI pain and fatigue sub-domains, respectively. PROMIS fatigue was highly correlated with pain interference (r=0.78) and negatively correlated with social participation (r=-0.71); pain interference was negatively correlated with social participation (r=-0.78) and physical function (r=-0.73) (Table 2). PROMIS anxiety and depression were highly correlated (r=0.80) with each other but not with other ESSPRI or PROMIS domains.
Conclusion: Conclusions. In our SS cohort, PROMIS pain interference and fatigue scores highly correlated with respective ESSPRI domains. Levels of PROMIS pain interference, fatigue, physical function, and anxiety were at least ½ SD worse than US population normative values. Pain interference and fatigue were not highly correlated with anxiety or depression. PROMIS instruments should be considered for use in SS research and clinical care. Future work will require validation in a larger cohort of SS patients and across broader demographics.
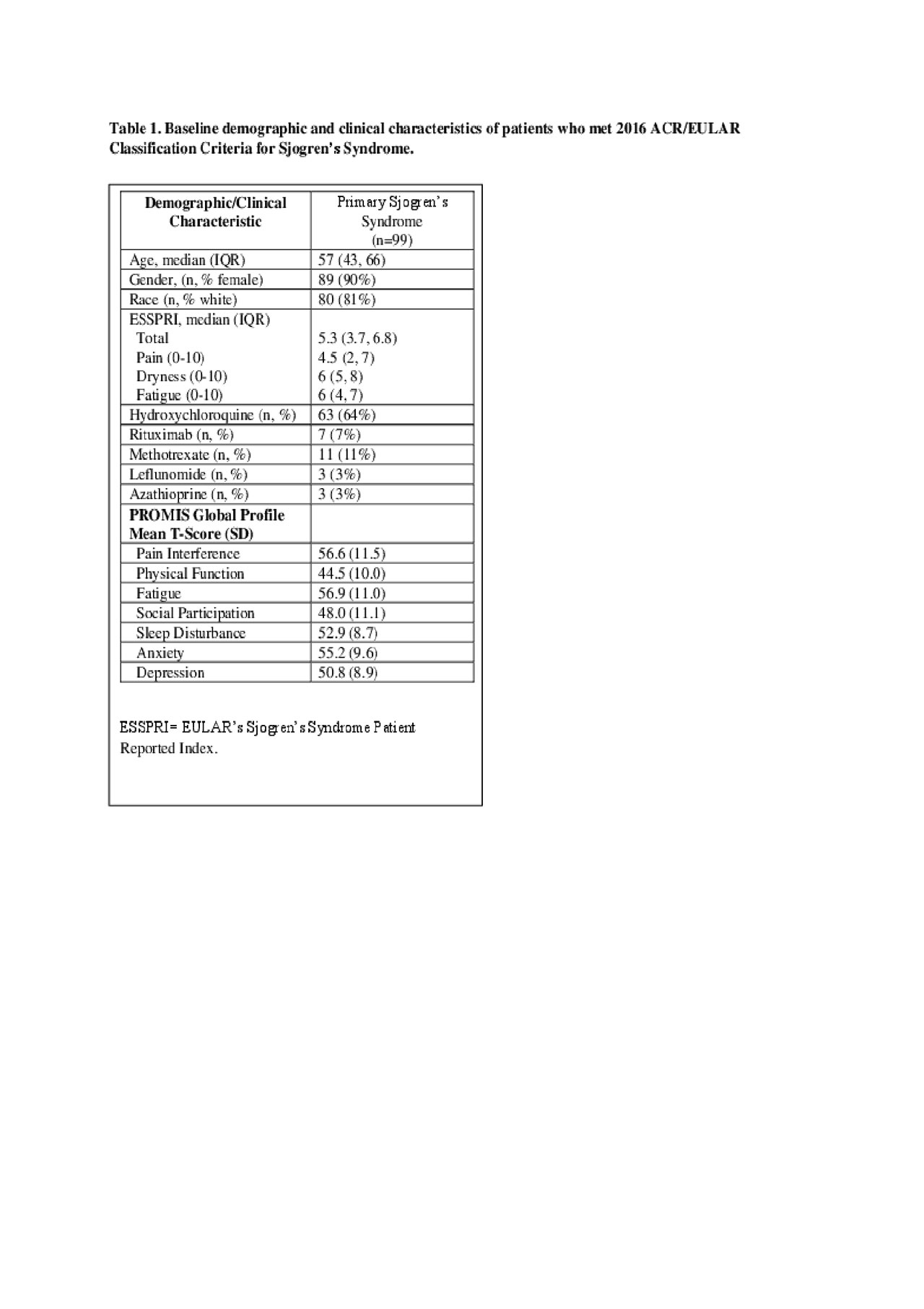
Sjogren’s ACR abstract Table 1
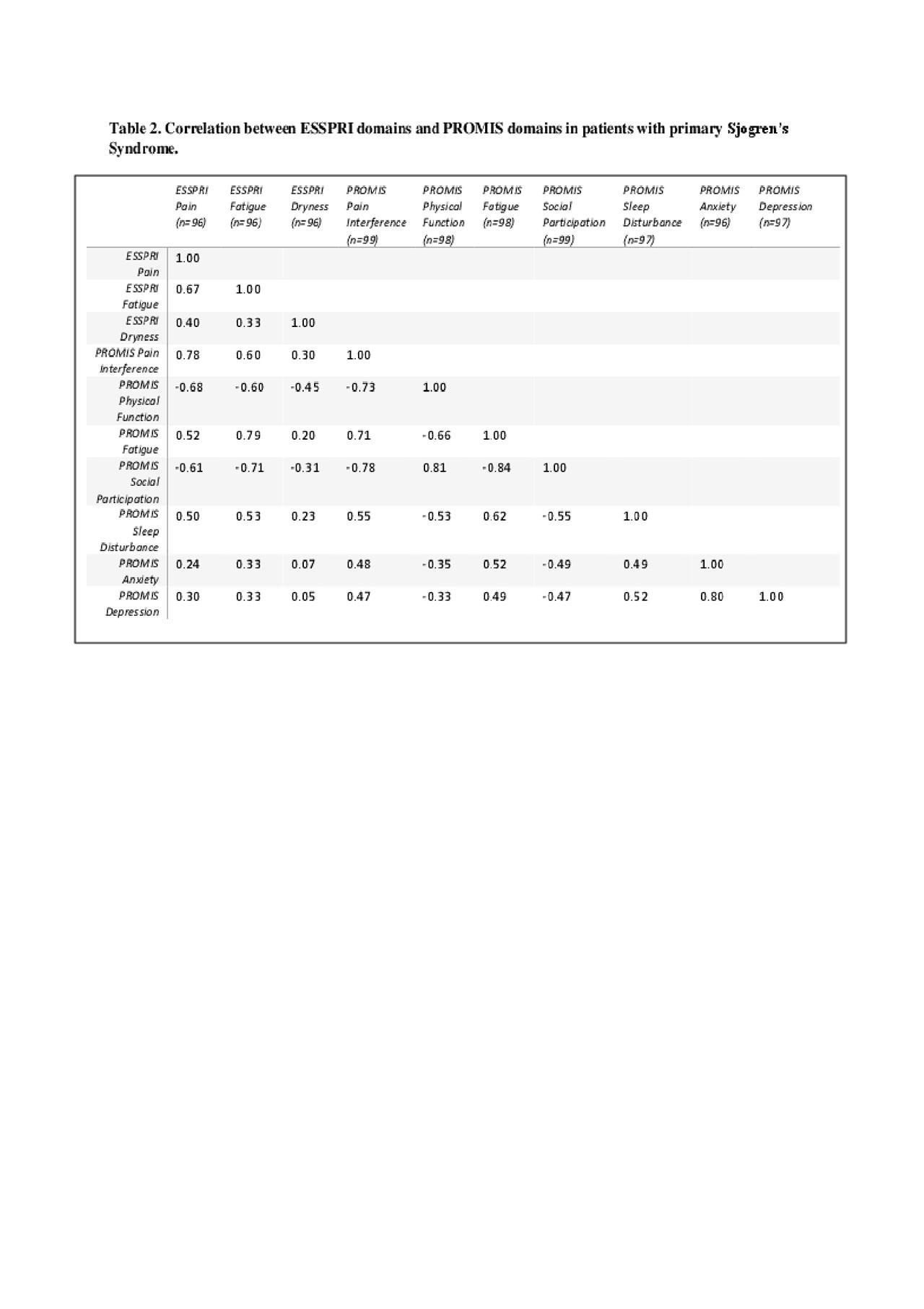
Sjogren’s ACR abstract Table 2
To cite this abstract in AMA style:
DiRenzo D, Robinson S, Bingham C, Grader-Beck T. Health-Related Quality of Life in Primary Sjogren’s Syndrome: Insights from Using PROMIS and Comparison to ESSPRI [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/health-related-quality-of-life-in-primary-sjogrens-syndrome-insights-from-using-promis-and-comparison-to-esspri/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-related-quality-of-life-in-primary-sjogrens-syndrome-insights-from-using-promis-and-comparison-to-esspri/
