Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile idiopathic arthritis (JIA) is a common, chronic rheumatic disease of childhood that carries substantial economic impact on patients (pts) and families1. The objective of this study was to evaluate the healthcare resource utilization and costs of JIA pts treated with abatacept (ABA) vs. other biologics and targeted disease modifying anti-rheumatic drugs (tDMARDs).
Methods: Pts (age < 18 years) from Truven’s MarketScan US Commercial Claims and Encounters claims database with ≥ 2 diagnoses of JIA (ICD-9: 714.3x or ICD-10: M08.xxx) separated by at least one day between 1 July 2006 till 31 March 2018 were split into 2 mutually exclusive cohorts of pts taking ABA vs. other tDMARDs as initial treatment on or after first JIA diagnosis. Index date was defined as the first prescription date for the drugs of interest. Healthcare resource use including inpatient, outpatient, emergency, urgent care visit and pharmacy visits and costs were calculated for the 6 months’ pre-index period and post-index period (index date till date of treatment switching, discontinuation, enrollment end or 183 days, whichever is earliest). The other tDMARDs pts were also stratified by TNF vs. non TNF pts. Statistical differences were assessed using chi-square and Kruskal-Wallis tests with significance level of 0.05.
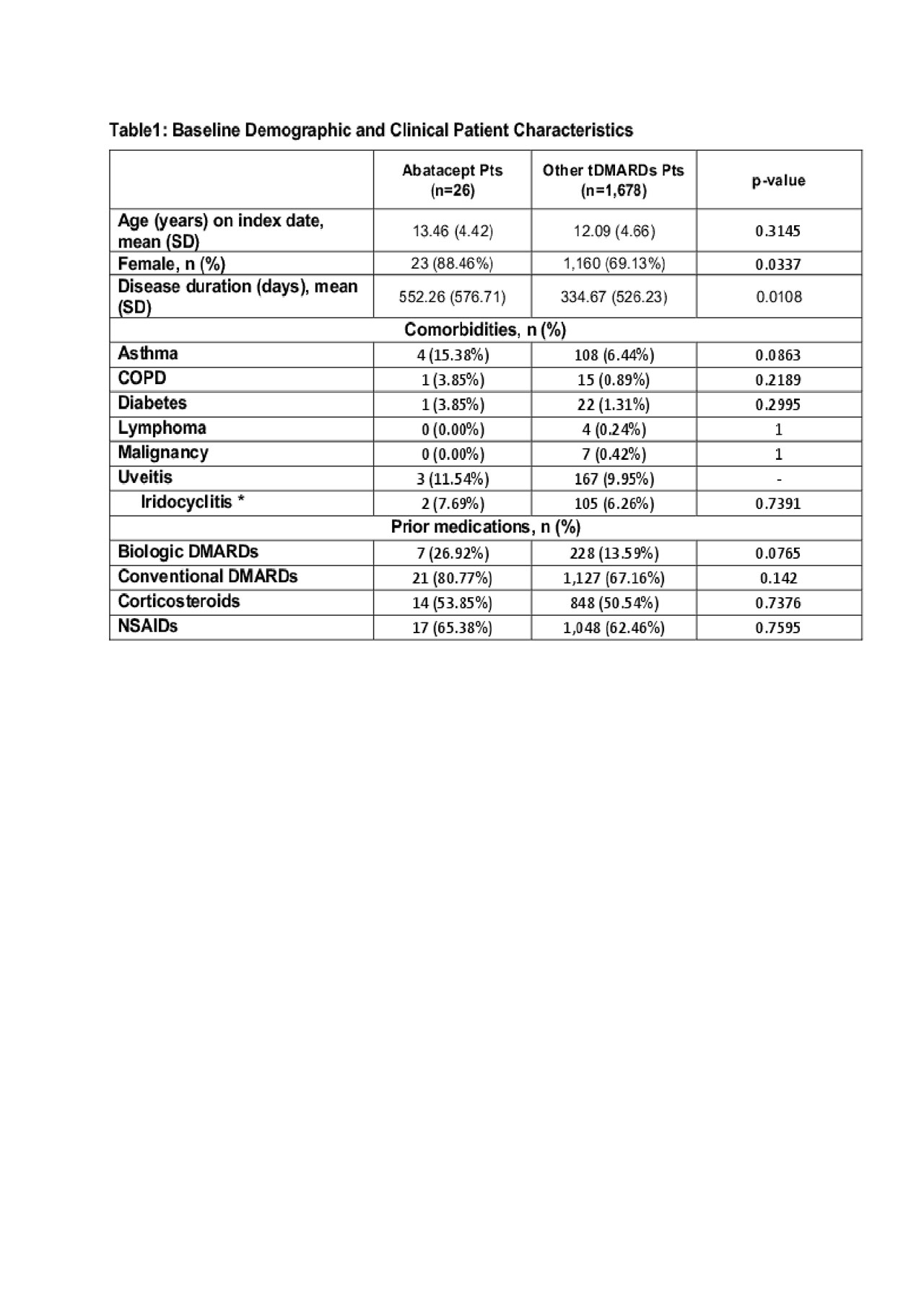
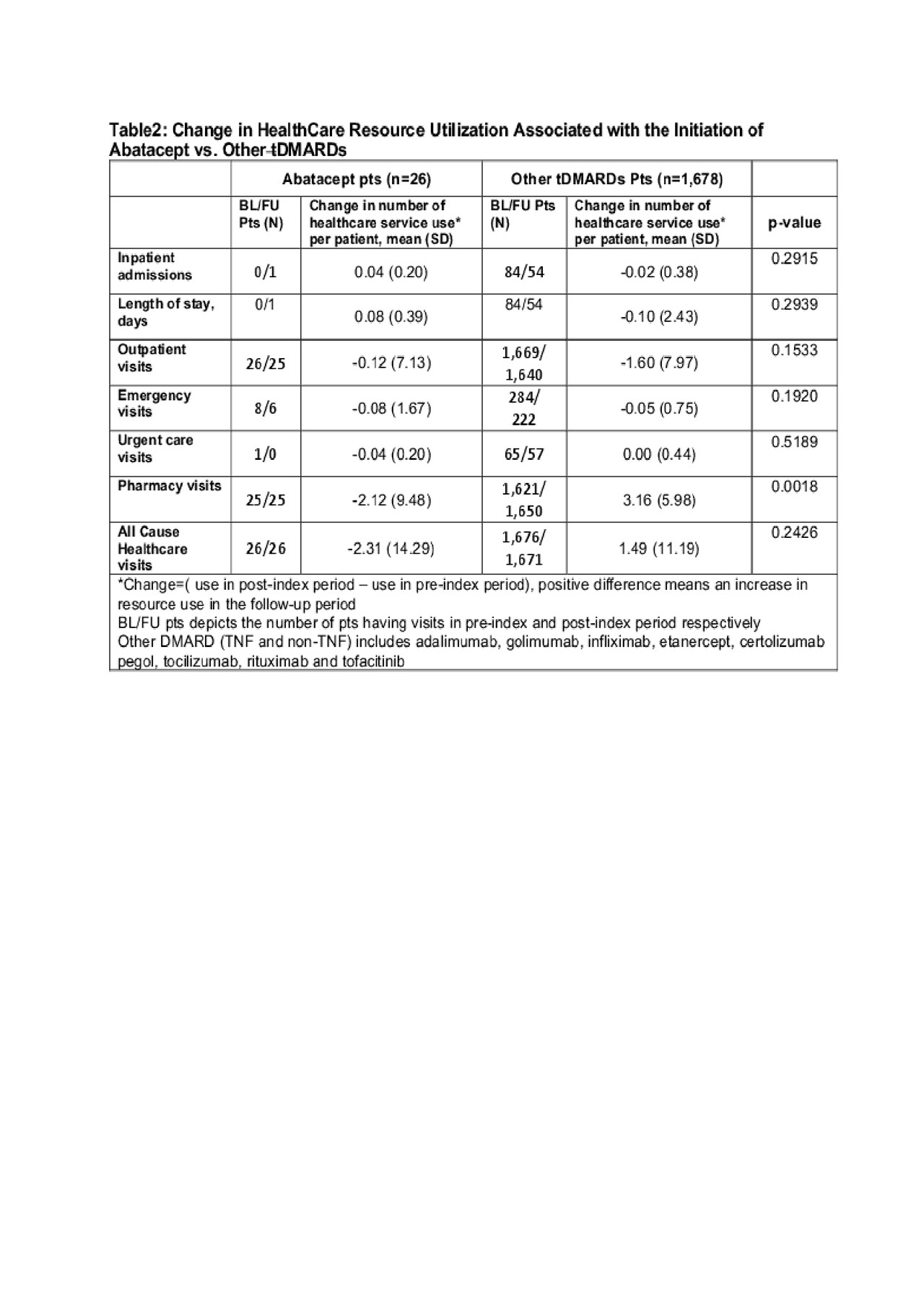
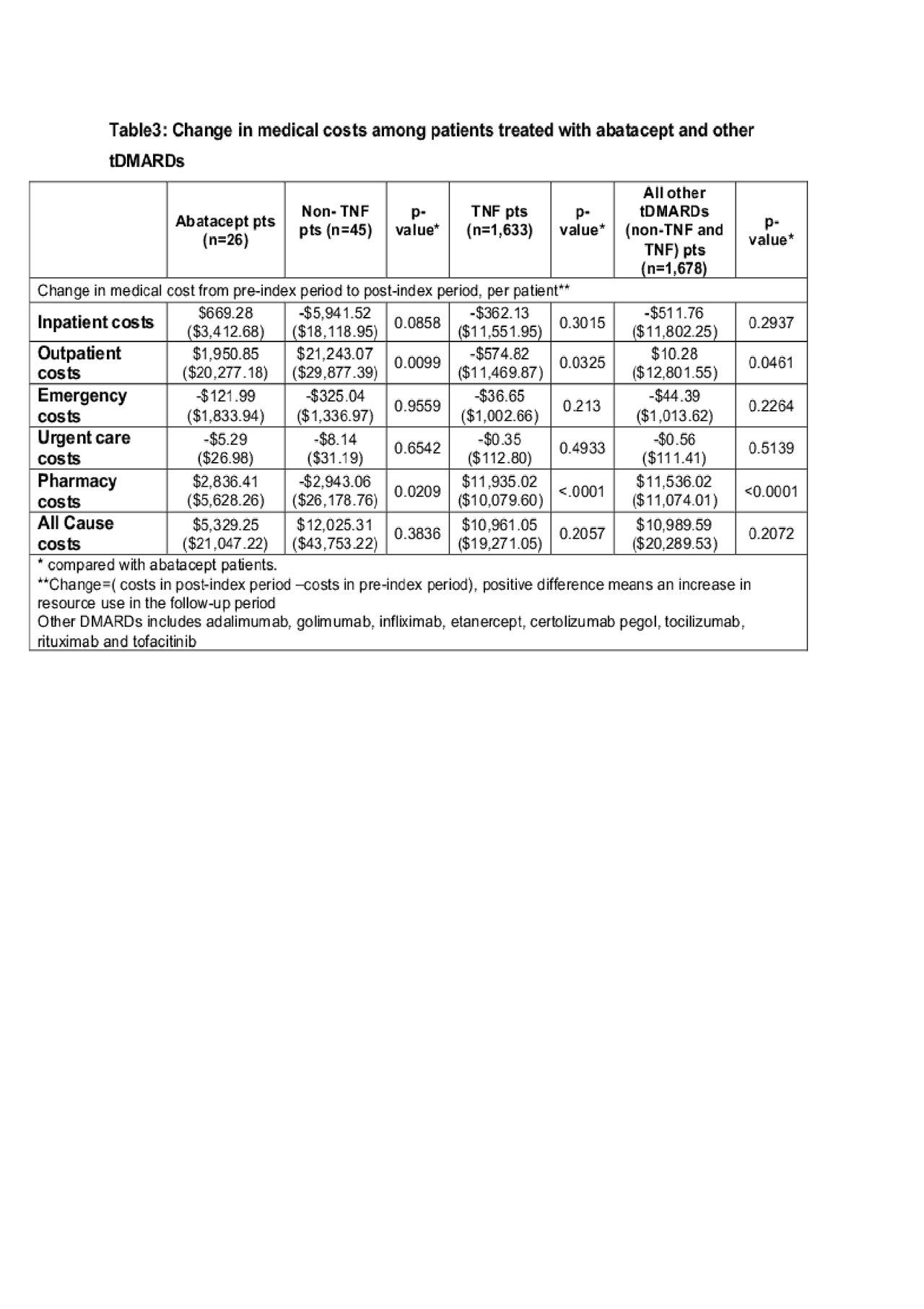
Results: A total of 1,704 pts (26 ABA and 1,678 biologics and other tDMARD pts) were included in the analysis. ABA pts are more likely to be female, have a longer disease duration and had more prior biologic use (Table 1). The number of total healthcare visit decreased by 2.31 per pt in ABA pts but increased by 1.49 per pt in other tDMARD pts. (Table 2) The difference was primarily driven by the change of pharmacy visits ( -2.12 per ABA pt vs. +3.16 per other tDMARDs pt) As a result, the increase in the pharmacy related costs for other tDMARDs pts was $5,660.3 more than that in ABA pts (Table 3). The increase of total cost over time was numerically greater in other tDMARD pts, TNF pts and non-TNF pts although the differences were not statistically significant.
Conclusion: Despite a longer disease duration, pts initiated with ABA had a greater decrease in pharmacy visit and related cost in comparison with other tDMARDs pts. Further analysis is warranted to understand the cause of the differences and its implications.
To cite this abstract in AMA style:
Zhuo J, Bao Y, Xia Q, Rao A, Sharma N, Han X, Wong R. Health Care Resource Utilization and Costs in Patients with Juvenile Idiopathic Arthritis Treated with Abatacept and Other Targeted Disease Modifying Anti-rheumatic Drugs [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/health-care-resource-utilization-and-costs-in-patients-with-juvenile-idiopathic-arthritis-treated-with-abatacept-and-other-targeted-disease-modifying-anti-rheumatic-drugs/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/health-care-resource-utilization-and-costs-in-patients-with-juvenile-idiopathic-arthritis-treated-with-abatacept-and-other-targeted-disease-modifying-anti-rheumatic-drugs/