Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is associated with an increased cardiovascular disease (CVD) burden, one that appears to be mitigated by increased concentrations of circulating HDL resulting from effective disease-modifying treatment. Our group has demonstrated that proteins modified with malondialdehyde-acetaldehyde (MAA), are present in the synovial tissues of RA patients and atherosclerotic lesions from non-RA patients with CVD. Previous data has also shown that MAA-modified proteins are bound and internalized by scavenger receptors (SRs) (SRA, SRBI, LOX-1, and CD36) present on immune cells. Therefore, in this study we evaluated whether HDL is modified by MAA and bound or internalized by monocytes.
Methods: Human HDL was labeled with 1,1′-dioctadecyl-3,3,3’3′-tetramethylindocarbocyanine perchlorate (DIL-HDL). For MAA modification DIL-HDL (1 mg/ml) was incubated with 2mM malondialdehyde and 1mM acetaldehyde for 3 days and fluorescence of the dihydropyridine structure determined. To evaluate receptor mediated binding and internalization, PMA activated THP-1 cells (human monocytic) were incubated on ice at either 4oC or 37oC (respectively) for 90 minutes with 25 μg/ml of DIL-Albumin, DIL-HDL and DIL-HDL-MAA. Cells were washed, fixed in paraformaldehyde, and subjected to flow cytometry at 594 nm wavelength. Analysis was performed using FlowJo V10. Data are expressed as percent positive compared to the DIL-labeled human albumin.
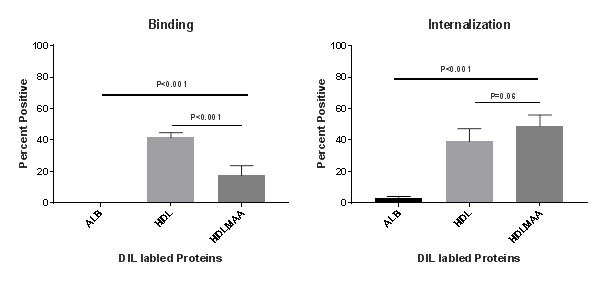
Results: Human HDL was modified at 30,000 fluorescent units (FU), which was greater than the MAA modification observed for human serum albumin (22,000 FU). As shown in the Figure, THP-1 cells bound HDL (41.73%) at the cell surface significantly better than HDL-MAA (17.55%; P<0.001). Internalization studies revealed that HDL-MAA (48.9%) was taken up more by the cells than HDL (39.14%) alone, although this difference did not achieve statistical significance.
Conclusion: These data show that HDL can be modified with the MAA adduct and HDL-MAA is subsequently bound and internalized by human monocytes. THP-1 cells contain multiple scavenger receptors capable of binding and internalizing altered self-ligands as a normal clearance mechanism. These results demonstrate that HDL modified with MAA is bound at lower levels that HDL alone, but is internalized more efficiently by human monocytes Taken together, these results suggest that MAA modification could impact cellular trafficking of HDL, thus influencing CVD pathogenesis in conditions characterized by oxidative stress and increased MAA adduct formation.
To cite this abstract in AMA style:
Duryee MJ, Clemens DL, Duryee LM, Easterling KC, Hunter CD, Klassen LW, O'Dell JR, Anderson DR, Mikuls TR, Thiele GM. HDL Modified with Malondialdehyde-Acetaldehyde (MAA) Is Bound and Rapidly Internalized By Human THP-1 Monocytes [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/hdl-modified-with-malondialdehyde-acetaldehyde-maa-is-bound-and-rapidly-internalized-by-human-thp-1-monocytes/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hdl-modified-with-malondialdehyde-acetaldehyde-maa-is-bound-and-rapidly-internalized-by-human-thp-1-monocytes/