Session Information
Date: Tuesday, November 9, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster III: Psoriatic Arthritis II (1801–1835)
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: In the DISCOVER-1 study, the anti-interleukin-23p19-subunit monoclonal antibody guselkumab (GUS) demonstrated robust efficacy across joint and skin clinical manifestations of psoriatic arthritis (PsA).1 Patients with PsA also experience a broad range of symptoms that negatively impact health-related quality of life (eg, pain, fatigue, anxiety, depression, sleep disturbance, poor physical function).2 This study assessed the treatment effect of GUS on general health outcomes in patients with PsA in the DISCOVER-1 trial through Week (W) 52 using the Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) instrument.
Methods: Patients with active PsA (≥3 swollen + ≥3 tender joints; C-reactive protein ≥0.3 mg/dL) and inadequate response to standard conventional therapies were randomized 1:1:1 to GUS 100 mg every 4 weeks (Q4W); GUS 100 mg at W0, W4, then Q8W; or placebo (PBO). PBO patients switched to GUS 100 mg Q4W at W24. PROMIS-29 contains 4 items for each of 7 domains (anxiety, depression, fatigue, pain interference, physical function, sleep disturbance, social participation) and 1 pain intensity item; 28 items are scored on a 5-point Likert-type scale, and pain intensity is rated from 0 to 10. The raw score of each domain is converted to a standardized T-score, with norms based on a general population mean score=50 and a standard deviation (SD)=10. Higher scores in anxiety, depression, fatigue, pain interference, and sleep disturbance indicate more severe symptoms; higher physical function and social participation scores indicate better health outcomes. Changes ≥5 points (1/2 SD of T-score) are considered clinically meaningful. Analyses were performed using both observed (mean scores/changes, effect sizes) and imputed (clinically meaningful response, whereby change from baseline was set to 0 at W24/52 for patients who had missing data or at W24 for patients who met treatment failure criteria prior to W24).
Results: At baseline, mean PROMIS-29 T-scores for physical function, social participation, sleep disturbance, pain, and fatigue were worse in the 381 PsA patients enrolled in DISCOVER-1 than in the general US population. Across all 7 domains, observed mean PROMIS-29 T-scores showed improvements in GUS-treated patients from baseline to W24 and W52 (Figure). Observed mean changes from baseline to W24 and W52, with calculated effect size, are shown (Table). In all patients, including those with imputed data, significantly higher percentages of patients in both GUS treatment groups vs PBO had ≥5-point improvements in fatigue, pain interference, physical function, sleep disturbance, social participation, and pain intensity domains at W24 (all nominal p< 0.05). Mean improvements in PROMIS-29 domains were maintained through W52.
Conclusion: In patients with active PsA, PROMIS-29 results indicate that GUS treatment was associated with clinically meaningful reductions in fatigue and pain and improvement in physical function and social participation, which were maintained through 1 year.
1. Deodhar A, et al. Lancet. 2020;395:1115-25
2. Orbai A, et al. Ann Rheum Dis. 2017;76:673-80
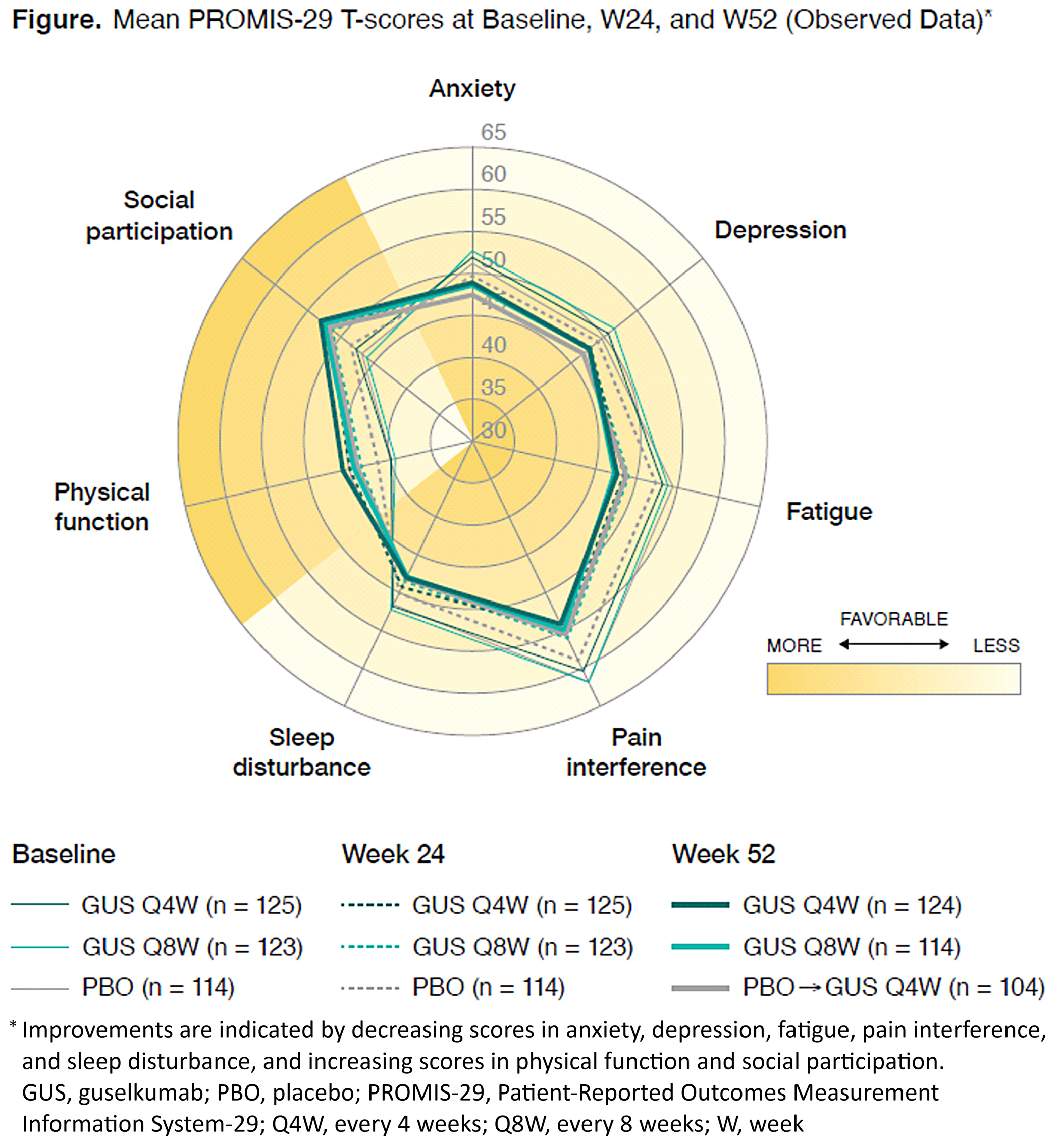 Figure. Mean PROMIS_29 T-scores at Baseline, W24, and W52 (Observed data)*
Figure. Mean PROMIS_29 T-scores at Baseline, W24, and W52 (Observed data)*
 Table. Mean Change and Effect Size of Change from Baseline in PROMIS_29 Domain Scores at W24 and W52 (Observed Data)
Table. Mean Change and Effect Size of Change from Baseline in PROMIS_29 Domain Scores at W24 and W52 (Observed Data)
To cite this abstract in AMA style:
Orbai A, Coates L, Deodhar A, Helliwell P, Ritchlin C, Kollmeier A, Hsia E, Xu X, Sheng S, Jiang Y, Liu Y, Han C. Guselkumab-Treated Patients with Psoriatic Arthritis Achieved Clinically Meaningful Improvements in General Health Outcomes Measured with PROMIS-29 Through 52 Weeks: Results from the Phase 3 DISCOVER-1 Trial [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/guselkumab-treated-patients-with-psoriatic-arthritis-achieved-clinically-meaningful-improvements-in-general-health-outcomes-measured-with-promis-29-through-52-weeks-results-from-the-phase-3-discover/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-treated-patients-with-psoriatic-arthritis-achieved-clinically-meaningful-improvements-in-general-health-outcomes-measured-with-promis-29-through-52-weeks-results-from-the-phase-3-discover/
