Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
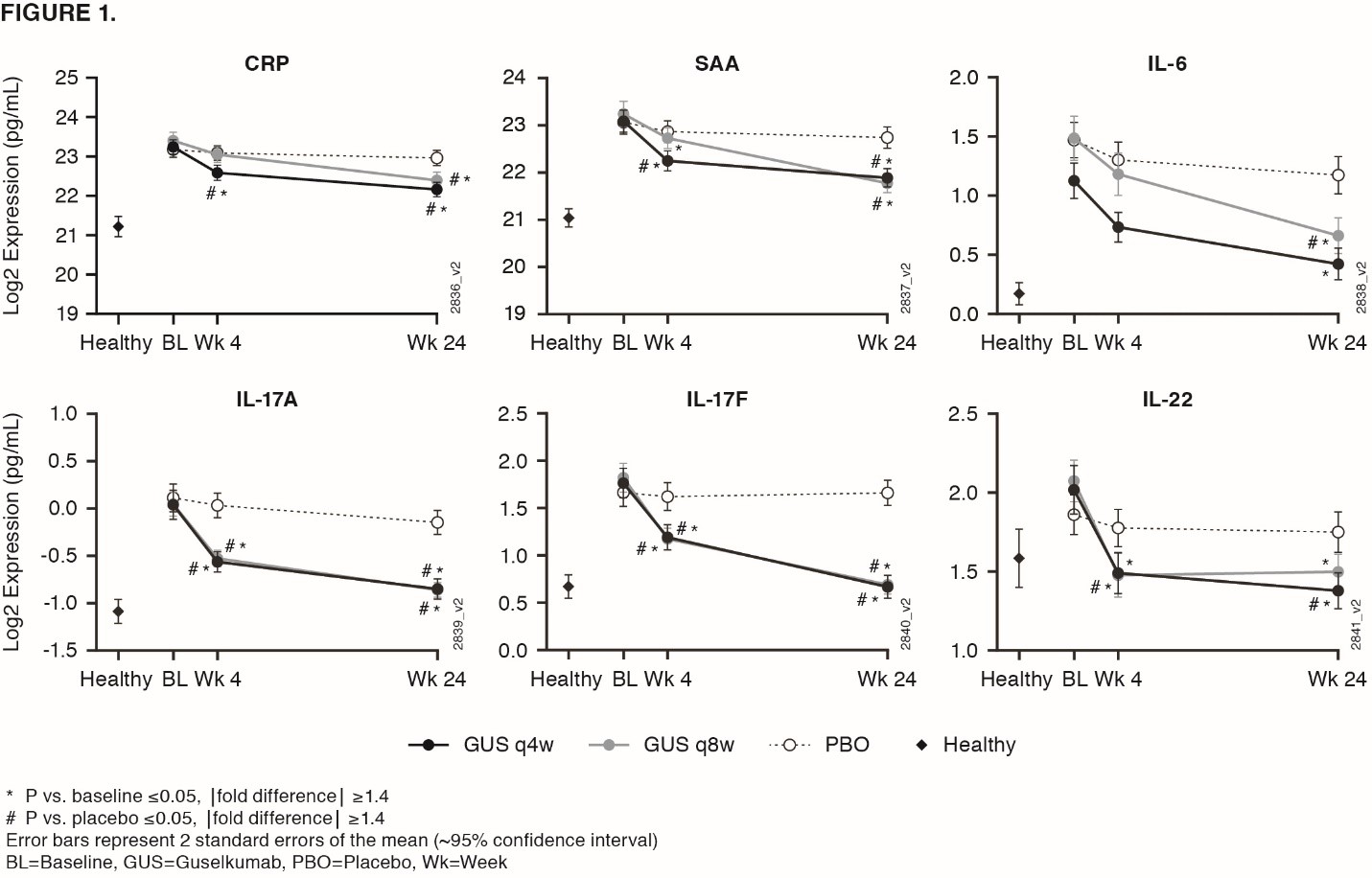
Background/Purpose: Guselkumab (GUS), an IL-23 inhibitor monoclonal antibody (MAb) that specifically binds the IL-23p19 subunit, demonstrated efficacy compared to placebo (PBO) in reducing skin & musculoskeletal signs & symptoms in patients (pts) with active PsA in two Ph3 studies, DISCOVER 1 & 2.1,2 Results from a GUS PsA Ph2 trial3 & Ustekinumab (UST, anti-IL12/23p40 MAb) PsA Ph3 trials (PSUMMIT 1 & 2)4 showed associations of baseline IL-17A, IL-17F, & CRP with baseline disease characteristics, & associations of GUS-induced cytokine reductions with clinical responses. We investigated cytokine expression in PsA & changes post GUS therapy.
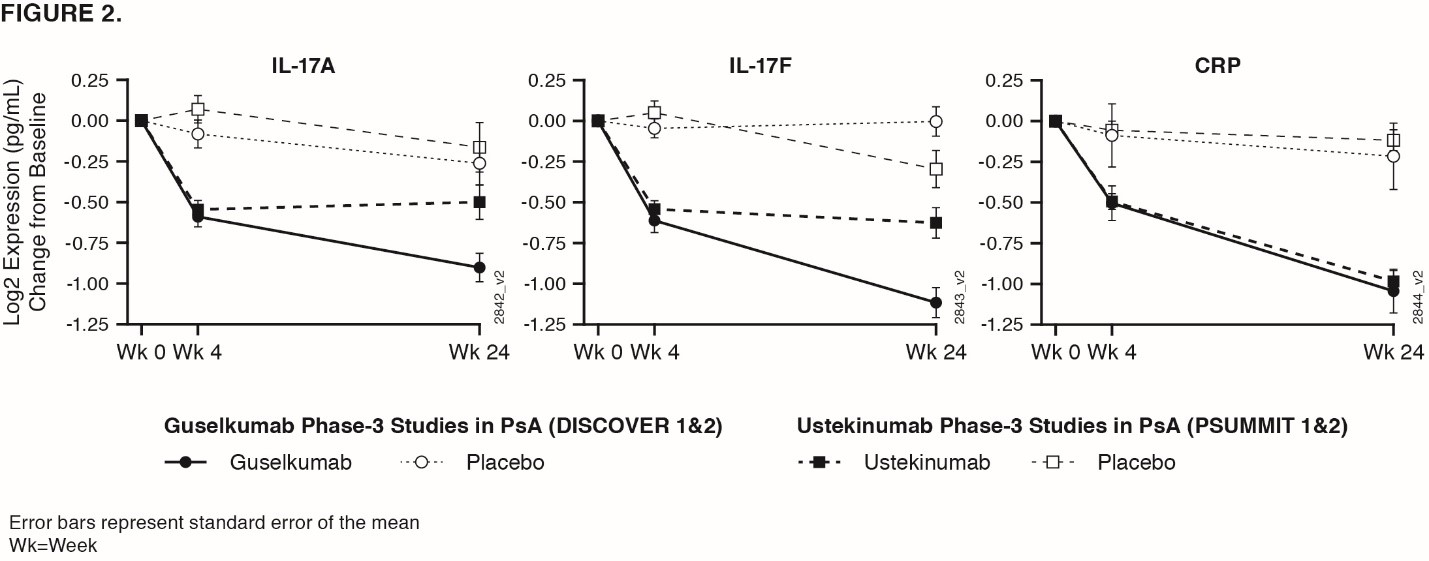
Methods: In DISCOVER 1 & 2, pts received GUS 100 mg at Week (Wk) 0, 4, then every 8Wks (q8w); GUS 100 mg q4w; or matching PBO. 21 serum biomarkers were measured in 300 PsA pts from DISCOVER at Wks 0, 4, & 24, & in 34 healthy controls matched for age, sex, & ethnicity. Serum proteins measured were acute phase reactants: CRP & SAA & inflammatory cytokines/chemokines: Th17 effector cytokines IL-17A, IL-17F, IL-22, soluble ICAM-1, soluble VCAM-1, IL-6, CXCL-8, IL-10, IL-13, IL-12p70, CCL22, IFN-γ, CCL2, CCL4, TNF-α, IL-1β, IL-2, IL-4 (MSD), & YKL-40. Serum IL-17A, IL-17B, & CRP measured in PSUMMIT4 were compared with GUS.
Results: Baseline serum levels of CRP, SAA, IL-6, IL-17A, & IL-17F were elevated in PsA pts vs healthy controls (p< 0.05, geometric mean [GM] ≥40% higher, FIG 1). No significant dysregulation in other cytokines in PsA pts vs healthy controls. Baseline IL-17A, IL-17F, IL-22, & CCL22 were significantly associated with baseline psoriasis disease activity (Body Surface Area & Psoriatic Area & Severity Index, Spearman Signed Rank p< 0.05, r >0.25). Baseline CRP, SAA, IL-6, & YKL40 were significantly associated with baseline joint disease (Disease Activity Score 28-CRP, Spearman p< 0.05, r >0.25). Baseline SAA, IL-6, IL-17A, & IL-17F were higher in pts with prior TNF inhibitor exposure than without (p< 0.05, GM ≥40% higher), although PsA pts with/without prior TNF inhibitor had higher levels vs healthy controls. GUS treatment resulted in decreases in serum CRP, SAA, IL-6, IL-17A, IL-17F, & IL-22 that were significantly greater than PBO as early as Wk 4 (FIG 1). These protein levels continued to decrease through Wk 24 in GUS-treated pts with both regimens (p< 0.05, GM decrease from baseline ≥ 33%). No significant difference in Wk 24 IL-17A & IL-17F levels for GUS vs healthy controls suggests a normalization of peripheral effector cytokines associated with the IL-23/Th17 axis post GUS treatment. Effects on IL-17A/IL-17F were greater in GUS vs UST treated pts, while CRP levels were similar in both programs (FIG 2).
Conclusion: Comprising a strong pharmacodynamic effect, GUS reduced serum protein levels of acute phase & Th17-effector cytokines (whose elevations at baseline were associated with PsA disease characteristics) & achieved comparable levels to those in healthy controls. In PsA pts, reductions of IL-17A & IL-17F by GUS were of greater magnitude than those by UST.
References:
- Deodhar et al. Arth Rheumatol 2019;71 S10: 1386
- Mease et al. Arth Rheumatol 2019;71 S10:5247
- Siebert et al. Ann Rheum Dis 2019;78 S2:293
- Siebert et al. Arth Rheumatol 2019;71:1660
To cite this abstract in AMA style:
Siebert S, McInnes I, Loza M, Ma K, Leander K, Lakshminarayanan V, Franks C, Cooper P, Sweet K. Guselkumab Induces Sustained Reduction in Acute Phase Proteins and Th17 Effector Cytokines in Active Psoriatic Arthritis in Two Phase-3 Clinical Trials (DISCOVER-1 and DISCOVER-2) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/guselkumab-induces-sustained-reduction-in-acute-phase-proteins-and-th17-effector-cytokines-in-active-psoriatic-arthritis-in-two-phase-3-clinical-trials-discover-1-and-discover-2/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-induces-sustained-reduction-in-acute-phase-proteins-and-th17-effector-cytokines-in-active-psoriatic-arthritis-in-two-phase-3-clinical-trials-discover-1-and-discover-2/