Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: DISCOVER 2 (DISC 2) is a Phase 3 trial of anti-IL-23-specific MAb Guselkumab (GUS) in PsA patients, who experience impaired physical function, resulting in disability, work productivity loss, and economic consequences.1 This study aimed to evaluate the effect of GUS on impaired work productivity and daily activity in DISC 2 using the Work Productivity and Activity Impairment Questionnaire: Psoriatic Arthritis (WPAI-PsA).
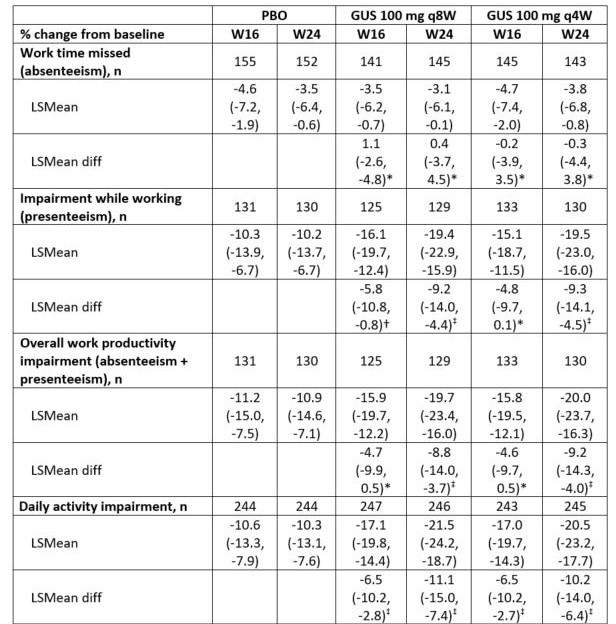
Methods: Bio-naïve adults with active PsA despite nonbiologic DMARDs &/or NSAIDs received subcutaneous GUS 100 mg every (q) 4 weeks (W); GUS 100 mg Week 0, Week 4, q8W; or placebo (PBO). WPAI-PsA assesses, due to PsA over the previous week, work time missed (absenteeism), impairment while working (presenteeism), and impaired overall work productivity (absenteeism + presenteeism) and daily activity. Percentage change from baseline was analyzed for WPAI-PsA domains using mixed-effect model repeated measure (MMRM). Indirect savings from improved overall work productivity were estimated with 2018 US mean yearly wage estimate (all occupations).2
Results: At Week 24, impaired overall work productivity and daily activity were improved 20-22% in GUS-treated and 10-11% in PBO-treated patients (Table). Potential yearly indirect savings from improved overall work productivity was $10,242 with GUS q8W and $10,404 with GUS q4W vs $5,648 with PBO; $4,594 and $4,756 difference, respectively.
Conclusion: Improvement in overall work productivity and daily activity was greater with GUS versus PBO among patients with moderate-to-severe PsA, resulting in potential annual incremental economic gains.
References:
- Tillett W et al. Rheumatol (Oxford). 2012; 51:275–283.
- US Bureau of Labor Statistics. May 2018 National Occupational Employment and Wage Estimates United States. https://www.bls.gov/oes/current/oes_nat.htm#00-000
To cite this abstract in AMA style:
Curtis J, McInnes I, Rahman P, Tillett W, Mease P, Kollmeier A, Hsia E, Zhou B, Agarwal P, Peterson S, Han C. Guselkumab Improved Work Productivity and Daily Activity in Patients with Psoriatic Arthritis: Results from a Phase 3 Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/guselkumab-improved-work-productivity-and-daily-activity-in-patients-with-psoriatic-arthritis-results-from-a-phase-3-trial/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-improved-work-productivity-and-daily-activity-in-patients-with-psoriatic-arthritis-results-from-a-phase-3-trial/