Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Many drugs are available in PsA and have demonstrated efficacy in randomized controlled trials (RCTs); however, real-world long-term data of drugs are scarce. PsABIOnd is a large, ongoing, global observational study in PsA. The aim of this interim analysis of the first ≥600 participants (pts) enrolled out of 1300 planned pts in PsABIOnd was to assess treatment persistence and achievement of clinical PsA outcomes at 6 months (M).
Methods: PsABIOnd (NCT05049798) is an ongoing observational study in PsA pts starting guselkumab (GUS) or IL‑17 inhibitors (i) as 1st-to-4th line of biologic therapy (monotherapy or in combination with other agents) per standard of care. The primary outcome is treatment persistence at 36M [1]. In this interim analysis, the subset of pts enrolled in the PsABIOnd study who had an assessment at the 6M visit (+/‑3M) were analysed according to their initial treatment, regardless of later switches. Persistence on treatment (i.e., no stop or switch) was assessed over 6M by treatment line via the Kaplan-Meier estimator function. Propensity score (PS) analysis was used to evaluate hazard ratio of stopping or switching GUS vs IL-17i prior to the 6M visit, adjusting for baseline (BL) variable imbalances across cohorts. Effectiveness was assessed at the 6M visit (descriptive unadjusted reports) by treatment line and included rates of achievement of Low Disease Activity (LDA)/remission (REM) by clinical Disease Activity Index for PsA (cDAPSA) and DAPSA (among pts with polyarticular PsA at BL), minimal disease activity (MDA; among non-MDA achievers at BL), psoriasis body surface area (BSA)< 3% (among pts with BSA≥3% at BL), and resolution of enthesitis by Leeds Enthesitis Index (LEI; among pts with LEI≥1 at BL) and dactylitis (among pts with dactylitis at BL).
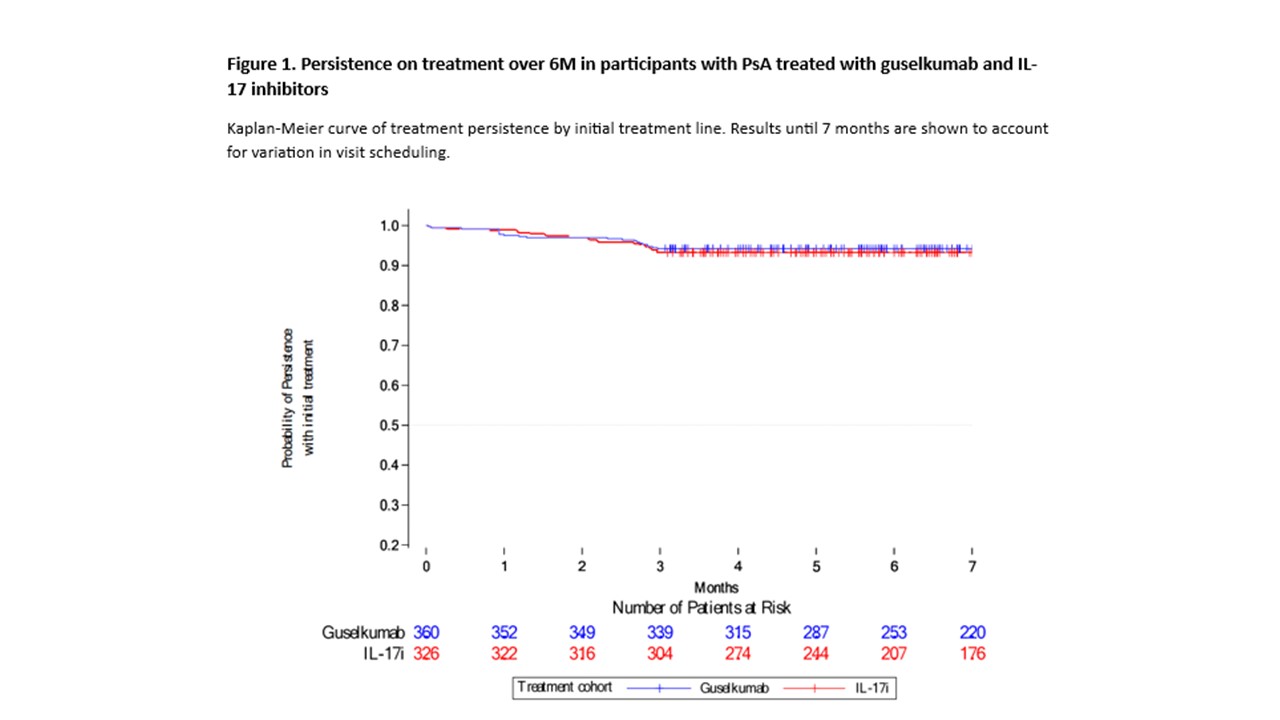
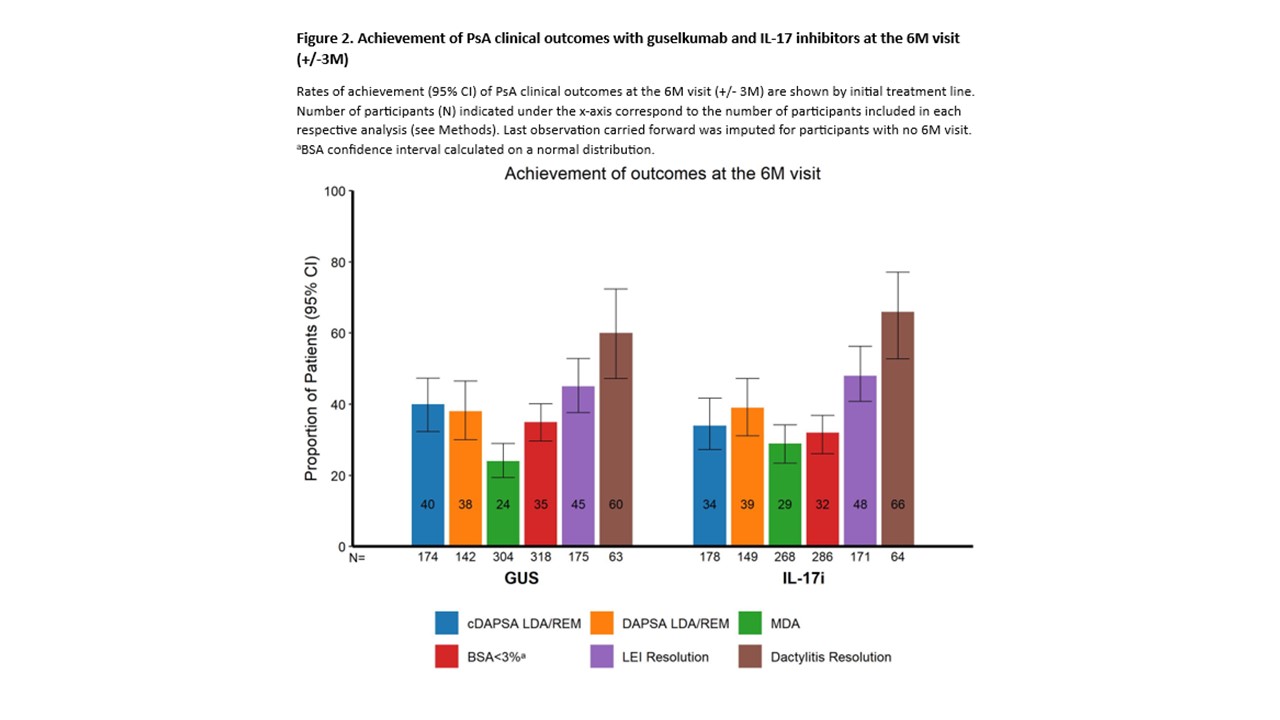
Results: As of 08-Jan-2024 (cutoff date), a total of 360 and 326 pts receiving GUS or IL-17i, respectively, as their initial treatment had follow-up data at the 6M visit: mean (GUS/IL-17i) age at BL was 52.0/53.6 years, and 63.1%/63.8% pts had previously received ≥1 targeted drug. The persistence on treatment at the 6M visit was high in both cohorts, with 339/360 (94.2%) GUS pts and 304/326 (93.3%) IL-17i pts remaining on their initial treatment line (PS-adjusted hazard ratio of GUS vs IL-17i stop/switch [95% confidence interval (CI)]: 0.87 [0.47-1.61]; Fig 1). Reasons for initial treatment line discontinuation were comparable between groups. Treatment effectiveness was similar for GUS vs IL-17i at the 6M visit (Fig 2), with similar rates (95% CI) of pts achieving cDAPSA LDA/REM (39.7% [32.3-47.3] vs 34.3% [27.3-41.7]); DAPSA LDA/REM (38.0% [30.0-46.5] vs 38.9% [31.1-47.2]); BSA< 3% (34.9% [29.7-40.1] vs 31.5% [26.1-36.9]); MDA (24.0% [19.4-29.0] vs 28.6% [23.4-34.2]); LEI resolution (45.1% [37.6-52.8] vs 48.5% [40.8-56.3]); and dactylitis resolution (60.3% [47.2-72.4] vs 65.6% [52.7-77.1]).
Conclusion: PsA pts had similar persistence on treatment with GUS or IL17i, and comparable rates of effectiveness across various PsA domains at 6M. These results provide additional information on real-world effectiveness and support efficacy data from RCTs.
References
1. Siebert. Rheumatol Ther 2023; 10:489-505
< 11.
To cite this abstract in AMA style:
Gossec L, Sharaf M, Baraliakos X, Kishimoto M, Lubrano E, Rahman P, Rampakakis E, Köleséri L, Koivunen M, Lavie F, Soriano E, Queiro Silva R, Behrens F, Siebert S. Guselkumab and IL-17 Inhibitors Show Comparable Treatment Persistence and Effectiveness in Psoriatic Arthritis: 6-month Interim Results of the PsABIOnd Observational Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/guselkumab-and-il-17-inhibitors-show-comparable-treatment-persistence-and-effectiveness-in-psoriatic-arthritis-6-month-interim-results-of-the-psabiond-observational-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-and-il-17-inhibitors-show-comparable-treatment-persistence-and-effectiveness-in-psoriatic-arthritis-6-month-interim-results-of-the-psabiond-observational-cohort-study/