Session Information
Session Type: Abstract Session
Session Time: 1:00PM-2:30PM
Background/Purpose: PsA leads to significant patient-perceived burden of disease. Targeted drugs have demonstrated efficacy in randomised controlled trials including in aspects of patient-reported impact. However, comparison data from observational studies are scarce, particularly for IL-23 and IL-17 inhibitors (i). As part of the 6 month (M) interim analysis of the first ≥600 participants (pts) enrolled in the PsABIOnd observational study of PsA, we assessed changes from baseline (BL) in PsA Impact of Disease-12 (PsAID‑12) following biologic treatment initiation.
Methods: PsABIOnd (NCT05049798) is an ongoing international, prospective, observational cohort study in 1300 planned PsA pts starting guselkumab (GUS) or IL-17i as first- to fourth-line of biologic therapy (monotherapy or in combination with other agents) per standard clinical practice [1]. All enrolled pts with available PsAID-12 data at BL and the 6M visit (+/-3M) were analysed according to their treatment group (regardless of later switches). Impact of PsA was assessed with PsAID-12 comprising 12 items (pain, fatigue, skin problems, work/leisure participation, function, discomfort, sleep, coping, fear, embarrassment, social participation and depression) scored 0‑10, with higher values indicating a worse state. Mean change from BL in PsAID-12 subdomain and total scores, and proportions of pts achieving minimal clinically important improvement (MCII, ≥1.4) at the 6M visit in both cohorts were determined. Propensity score (PS) analysis evaluated treatment effect for the change in PsAID-12 total score and MCII (using nonresponder imputation), adjusting for BL variable imbalances across cohorts. Subdomain analyses were descriptive.
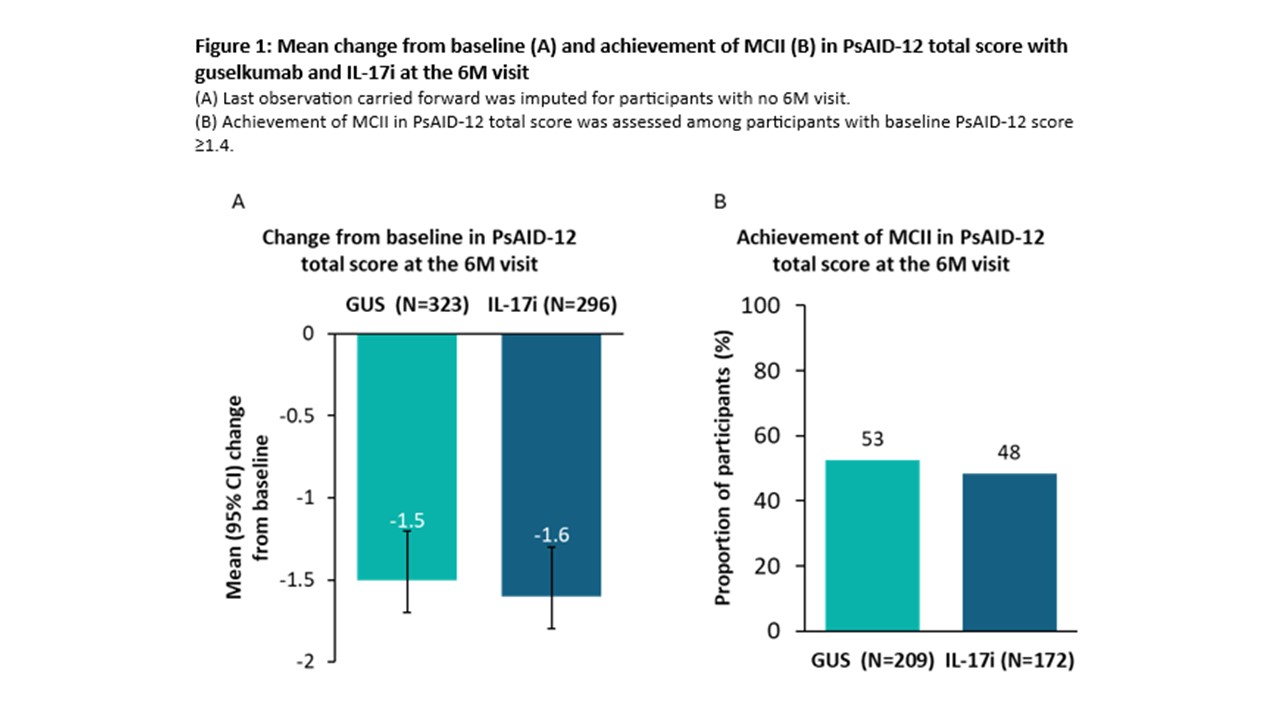
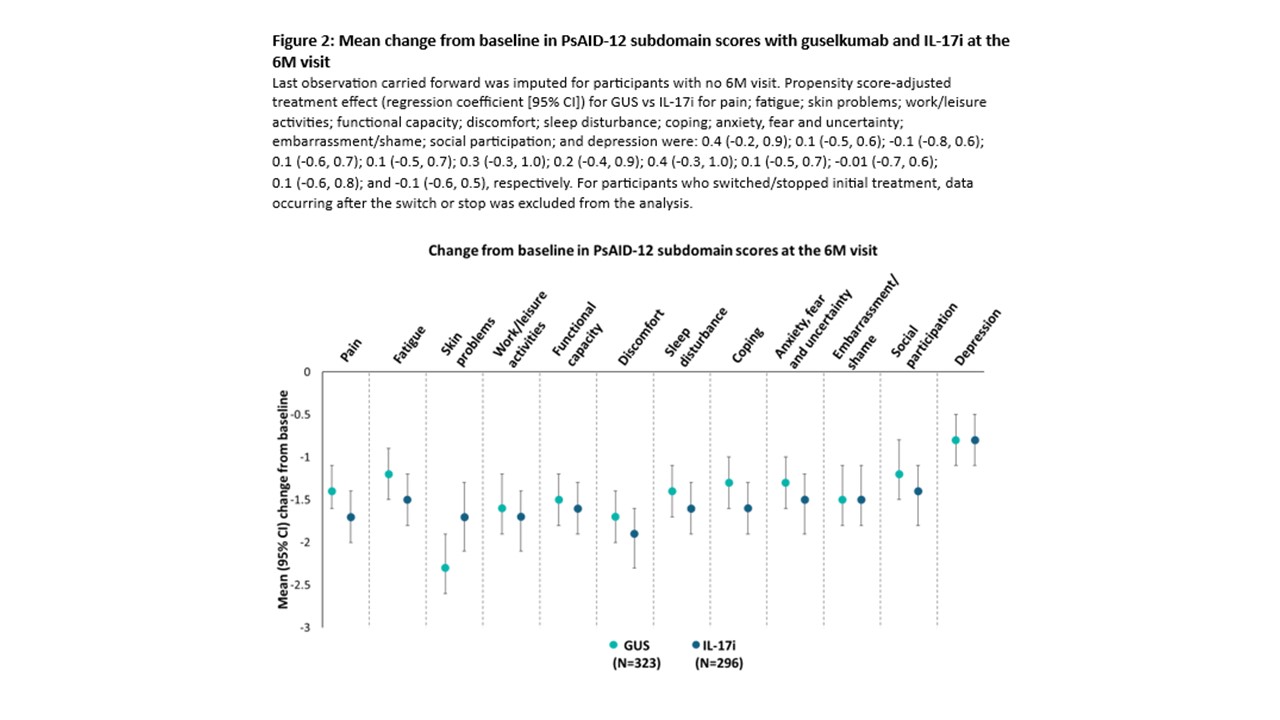
Results: At the Jan 2024 cutoff date, 323 and 296 pts receiving GUS or IL-17i, respectively, with PsAID-12 data available at BL and the 6M visit were analysed. In both cohorts, PsAID-12 subdomains with highest impact at BL were pain, fatigue, and discomfort. At the 6M visit, mean (95% confidence interval [CI]) changes from BL in PsAID-12 total score were similar in the GUS (-1.5 [-1.7; -1.3]) and IL-17i (-1.6 [‑1.8; ‑1.3]) cohorts (Fig 1A). PS-adjusted treatment effect (regression coefficient [95% CI]) for GUS vs IL‑17i in change from BL in PsAID-12 total score was not significant (0.2 [-0.3; 0.6]). Proportions of pts achieving MCII in PsAID-12 total score at the 6M visit were 53% and 48% in the GUS and IL-17i cohorts, respectively (Fig 1B), with a non-significant PS-adjusted treatment effect (odds ratio [95% CI]: 1.2 [0.8; 1.8]). At the 6M visit, mean changes from BL in subdomain scores were similar across cohorts, ranging from ‑0.8 (-1.1; -0.5) for depression to -2.3 (-2.7; -2.0) for skin problems in the GUS cohort, and ‑0.8 (-1.1; -0.5) for depression to ‑1.9 (-2.2; -1.5) for discomfort in the IL-17i cohort (Fig 2).
Conclusion: By 6M of treatment, clinically meaningful improvements in PsAID-12 total score were seen in around half of pts treated with GUS or IL-17i, with similar magnitudes of effect across subdomains in both cohorts. These results may be useful in shared treatment decision-making.
References
1. Siebert. Rheumatol Ther 2023; 10:489
To cite this abstract in AMA style:
Siebert S, Sharaf M, Selmi C, Rahman P, Kishimoto M, Soriano E, Rampakakis E, Köleséri L, Koivunen M, Lavie F, Queiro Silva R, Behrens F, Lubrano E, Gossec L. Guselkumab and IL-17 Inhibitors Improve Patient-perceived Impact of Psoriatic Arthritis Similarly: 6-month Interim Results of the PsABIOnd Observational Cohort Study [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/guselkumab-and-il-17-inhibitors-improve-patient-perceived-impact-of-psoriatic-arthritis-similarly-6-month-interim-results-of-the-psabiond-observational-cohort-study/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-and-il-17-inhibitors-improve-patient-perceived-impact-of-psoriatic-arthritis-similarly-6-month-interim-results-of-the-psabiond-observational-cohort-study/