Session Information
Date: Sunday, November 10, 2019
Title: 3S032: Plenary I (805–809)
Session Type: Plenary Session I
Session Time: 11:00AM-12:30PM
Background/Purpose: Guselkumab (GUS), an anti-interleukin-23p19 monoclonal antibody, is approved to treat PsO. We evaluated GUS efficacy and safety in a Phase 3, double-blind, PBO-controlled trial in pts with active PsA who were biologic-naïve or prior TNFα inhibitor (TNFi)-treated (DISCOVER-1).
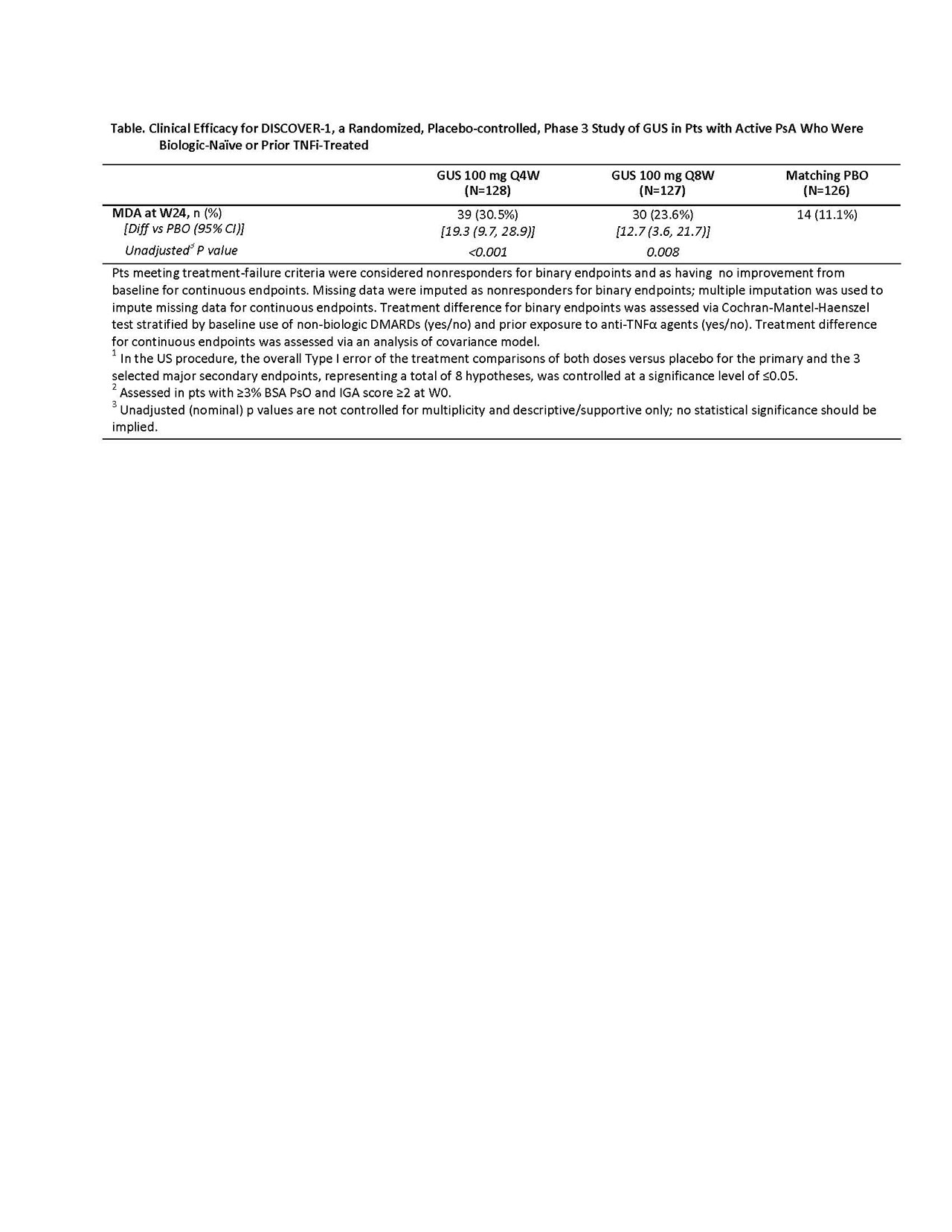
Methods: Adults with active PsA (≥3 swollen+≥3 tender joints; CRP ≥0.3mg/dL) despite standard therapies (eg, non-biologic DMARDs, apremilast, or NSAIDs) were eligible. Approx. 30% of pts previously could have received or have had inadequate response to 1-2 TNFi. Pts were randomized 1:1:1, stratified by Week [W] 0 DMARD use [Y/N] and prior TNFi use (Y/N), to GUS 100mg Q4W; GUS 100mg at W0, W4, Q8W (Q8W); or PBO. Concomitant stable use of select non-biologic DMARDs, oral corticosteroids, and NSAIDs was allowed. At W16, pts with < 5% improvement in tender+swollen joints could initiate or increase the dose of permitted medications while continuing study treatment. The primary endpoint was ACR20 at W24. Major secondary endpoints included: Investigator’s Global Assessment (IGA) PsO response (IGA=0/1 + ≥2-grade reduction) at W24 in pts with ≥3% BSA PsO & IGA ≥2 at W0; changes in DAS28-CRP, HAQ-DI and SF-36 PCS scores and ACR50/70 response at W24; and ACR20/50 response at W16. As preplanned, enthesitis or dactylitis data were pooled with those from the companion Phase 3 GUS in PsA study (DISCOVER-2; to be reported elsewhere). Due to regional health authority differences in regulatory requirements for multiplicity control, two multiplicity control procedures were prespecified (Global and US). Results of statistical testing via US procedures are presented. Unadjusted (nominal) p-values are provided for other endpoints (Table). Adverse events (AEs) through W24 are reported. Results: 381 pts were treated and analyzed; baseline characteristics were consistent with moderate-to-severe disease (mean BSA involved with PsO: 13.4%, pts with IGA=3-4: 42.5%; mean swollen/tender joint counts: 9.8/19.3). Significantly more pts receiving GUS Q4W (58.6%) and Q8W (52.8%) vs PBO (22.2%, both p< 0.001) achieved ACR20 response at W24 (Figure). Consistent response rates were observed in the subgroups of pts with or without prior TNFi use (Table). Significantly greater improvements in HAQ-DI and SF-36 PCS scores were seen in GUS- vs PBO-treated pts from W0 to W24. Among 249 pts with ≥3% BSA PsO and IGA ≥2 at W0, significantly more GUS- vs PBO-treated pts achieved IGA response. Higher proportions of pts achieved ACR20 response at W16, ACR50 response at W16/24, ACR70 response at W24, and PASI75/90/100 responses at W24. More GUS Q4W- or Q8W- vs PBO-treated pts achieved MDA response at W24 (Table). Serious AEs, serious infections, and death occurred in 9/381 (2.4%), 2/381 (0.5%), and 1/381 (0.3%) pts, respectively. Conclusion: In pts with active PsA who were biologic-naïve or had been treated with TNFi, both GUS Q4W and Q8W demonstrated efficacy for joint and skin symptoms, physical function, and quality of life relative to PBO. Observed AEs were consistent with GUS safety established in PsO.
To cite this abstract in AMA style:
Deodhar A, Helliwell P, Boencke W, Hsia E, Kollmeier A, Subramanian R, Xu X, Sheng S, Zhou B, Ritchlin C. Guselkumab, an Anti-interleukin-23p19 Monoclonal Antibody, in Patients with Active Psoriatic Arthritis Who Were Biologic-Naïve or Prior TNFα Inhibitor-Treated: Week 24 Results of a Phase 3, Randomized, Double-blind, Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/guselkumab-an-anti-interleukin-23p19-monoclonal-antibody-in-patients-with-active-psoriatic-arthritis-who-were-biologic-naive-or-prior-tnf%ce%b1-inhibitor-treated-week-24-results-of-a-phase-3-rando/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-an-anti-interleukin-23p19-monoclonal-antibody-in-patients-with-active-psoriatic-arthritis-who-were-biologic-naive-or-prior-tnf%ce%b1-inhibitor-treated-week-24-results-of-a-phase-3-rando/