Session Information
Session Type: Late-Breaking Abstract Poster Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Guselkumab (GUS), an anti-interleukin-23p19 monoclonal antibody, is approved for psoriasis (PsO). We assessed GUS efficacy and safety in DISCOVER-1 (ACR2019 Abstract ID697955) and DISCOVER-2, two Phase 3 trials in psoriatic arthritis (PsA).
Methods: In DISCOVER-2, adults with active PsA (≥5 swollen+≥5 tender joints; CRP ≥0.6mg/dL) despite non-biologic DMARDs and/or NSAIDs (biologic-naïve) were randomized (1:1:1) to GUS 100mg every 4 wks (Q4W); GUS 100mg at W0, W4, Q8W (Q8W); or placebo (PBO). Concomitant stable select non-biologic DMARDs, oral corticosteroids, and NSAIDs were allowed. At W16, patients (pts) with < 5% improvement in tender+swollen joints could initiate/increase the dose of permitted medications. The primary endpoint was W24 ACR20 response. Major secondary endpoints (MSEs) at W24 were Investigator’s Global Assessment (IGA) PsO response (IGA=0/1 and ≥2-grade reduction) in pts with ≥3% BSA PsO&IGA ≥2 at W0; changes in HAQ-DI, PsA-modified van der Heijde-Sharp (vdH-S), and SF-36 PCS/MCS scores; resolution of enthesitis/dactylitis (using pooled DISCOVER-1&2 data); change in DAS28-CRP; and ACR50/70 responses. MSEs at W16 were ACR20/50 responses. Multiplicity-adjusted p-values for controlled endpoints, and nominal (unadjusted) p-values for uncontrolled endpoints, are presented. Adverse events (AEs) through W24 are reported.
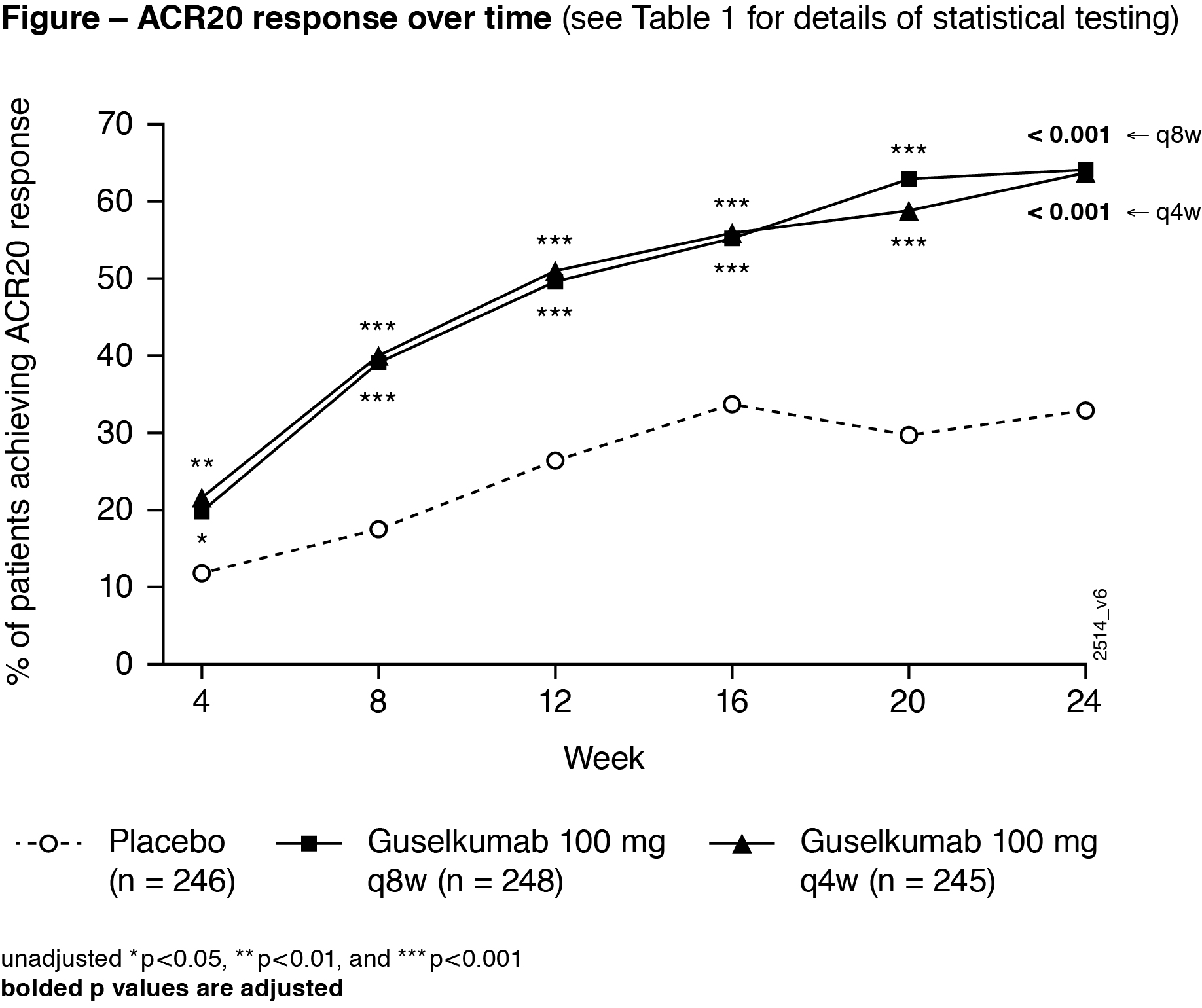
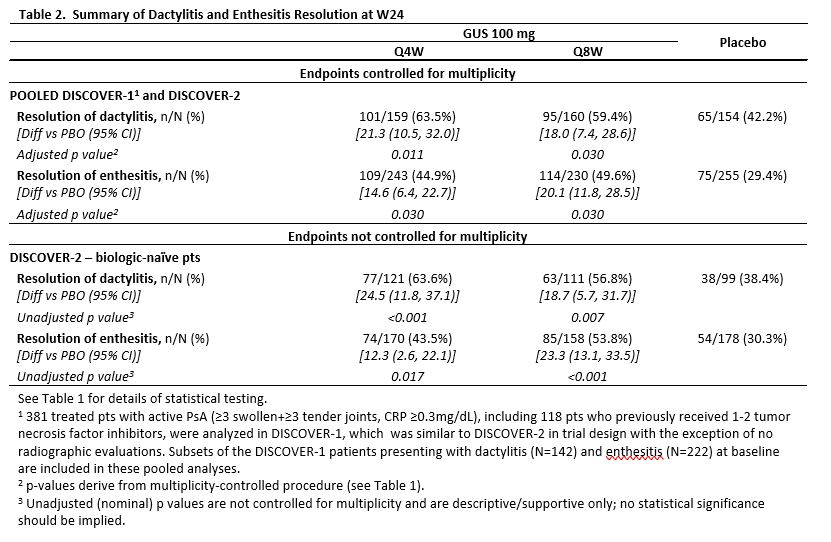
Results: 739 treated pts in DISCOVER-2 with moderate-to-severe disease (mean swollen/tender joints: 12/21, median CRP: 1.2 mg/dL, mean BSA with PsO: 17.4%, IGA=3 or 4: 46.1% of pts) were analyzed. Significantly more GUS Q4W (63.7%) and Q8W (64.1%) vs PBO (32.9%) pts achieved ACR20 response at W24 (both adjusted p< 0.001). Both GUS doses separated from PBO by W4 (Figure). Among pts with ≥3% BSA PsO&IGA ≥2 at W0, significantly more GUS Q4W and Q8W vs PBO pts achieved IGA response at W24 (both adjusted p< 0.001). Significantly greater improvements from baseline in HAQ-DI (adjusted p< 0.001) and SF-36 PCS (adjusted p≤0.011) were seen with GUS Q4W and Q8W vs PBO at W24. Mean changes in total modified vdH-S scores at W24 were significantly lower for GUS Q4W (0.29) and numerically lower for GUS Q8W (0.52) vs. PBO (0.95; adjusted p=0.011 and p=0.072, respectively). Numerically larger mean improvements in SF-36 MCS scores were seen with GUS Q4W (4.22) and Q8W (4.17) than PBO (2.14; both adjusted p=0.072; Table 1). Among pooled DISCOVER-1&2 pts with the condition at baseline, significantly higher proportions of GUS Q4W and Q8W vs PBO pts had resolved enthesitis or dactylitis at W24 (all adjusted p< 0.05; Table 2). Numerically higher proportions of GUS Q4W and Q8W than PBO pts with PASI75/90/100 (among pts with ≥3% BSA PsO&IGA ≥2 at W0) and MDA responses at W24 (Table 1) were observed. Serious AEs and serious infections occurred in 18/739 (2.4%) and 5/739 (0.7%) pts, respectively, and no pt died through W24.
Conclusion: In pts with active PsA, GUS Q4W and Q8W significantly improved joint and skin symptoms, physical function, and quality of life, and resolved enthesitis/dactylitis. GUS Q4W significantly reduced radiographic damage progression vs. PBO. GUS was well tolerated, and observed AEs were consistent with GUS safety in PsO pts.
To cite this abstract in AMA style:
Mease P, Rahman P, Gottlieb A, Hsia E, Kollmeier A, Xu X, Subramanian R, Agarwal P, Sheng S, Zhou B, van der Heijde D, McInnes I. Guselkumab, an Anti-interleukin-23p19 Monoclonal Antibody, in Biologic-naïve Patients with Active Psoriatic Arthritis: Week 24 Results of the Phase 3, Randomized, Double-blind, Placebo-controlled Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/guselkumab-an-anti-interleukin-23p19-monoclonal-antibody-in-biologic-naive-patients-with-active-psoriatic-arthritis-week-24-results-of-the-phase-3-randomized-double-blind-placebo-controlled-stud/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guselkumab-an-anti-interleukin-23p19-monoclonal-antibody-in-biologic-naive-patients-with-active-psoriatic-arthritis-week-24-results-of-the-phase-3-randomized-double-blind-placebo-controlled-stud/