Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The purpose of this systematic review was to identify existing guidelines for antimalarial prescribing and monitoring in rheumatic diseases, specifically for hydroxychloroquine, and how these guidelines compare between organizations and have evolved over time.
Methods: A literature search was conducted using Embase and Medline to identify guidelines published from 1946 to September 2018. MeSH terms were employed for the search strategy with alternative spelling and related words entered as keywords and separated by ‘OR’ to broaden results (Figure 1). The Embase and Medline strategies both contained the same sub-searches for antimalarials and retinal disease, however they differed in the use of MeSH terms pertaining to guidelines. In addition to reviewing all English search results, references of all articles were reviewed to retrieve additional guidelines.
Results: A total of 243 results were reviewed, after accounting for duplicates, to obtain 11 recommendations. The American Academy of Ophthalmology, Royal College of Ophthalmologists and Canadian editorials summarize ophthalmology recommendations. (Table 1) Rheumatology sources include American College of Rheumatology and Canadian Rheumatology Association statements. (Table 2) American and British guidelines changed from suggesting hydroxychloroquine doses ≤6.5 mg/kg/day to ≤5 mg/kg/day more recently. American guidelines recommended baseline visual field testing and annual screening after five years. Visual field testing evolved from using Amsler grids to current recommendations of 10-2 automated visual fields and spectral-domain optical coherence tomography (SD-OCT). The 2012 Canadian recommendations suggested initial field testing every two years, with SD-OCT after 10 years. Older British guidelines advocated for baseline and annual assessment with Amsler grids during rheumatology clinic visits. The 2018 British guidelines support baseline and annual screening after five years with 10-2 visual fields, SD-OCT and fundus autofluorescence.
Conclusion: The newest recommendations suggest a hydroxychloroquine dose of ≤5 mg/kg/day. Retinal toxicity is irreversible; and the risk increases over time on antimalarial therapy. Annual screening after five years of treatment with automated visual fields and SD-OCT seems to be warranted to detect early changes and discontinue therapy if necessary. It is uncertain whether the magnitude of retinal toxicity is increasing due to early detection with more sensitive tests or if newer recommendations are based on best evidence.

Figure 1- Embase and Medline Search Strategies
.mp: Multiple Posting; *: Truncation Symbol; /: Subject Heading; .pt: Publication Type

Table 1. International Ophthalmology Guidelines on Antimalarial Use
HCQ: Hydroxychloroquine; CQ: Chloroquine; VF: Visual Fields; SD-OCT: Spectral-Domain Optical Coherence Tomography; mfERG: Multifocal Electroretinography; FAF: Fundus Autofluorescence
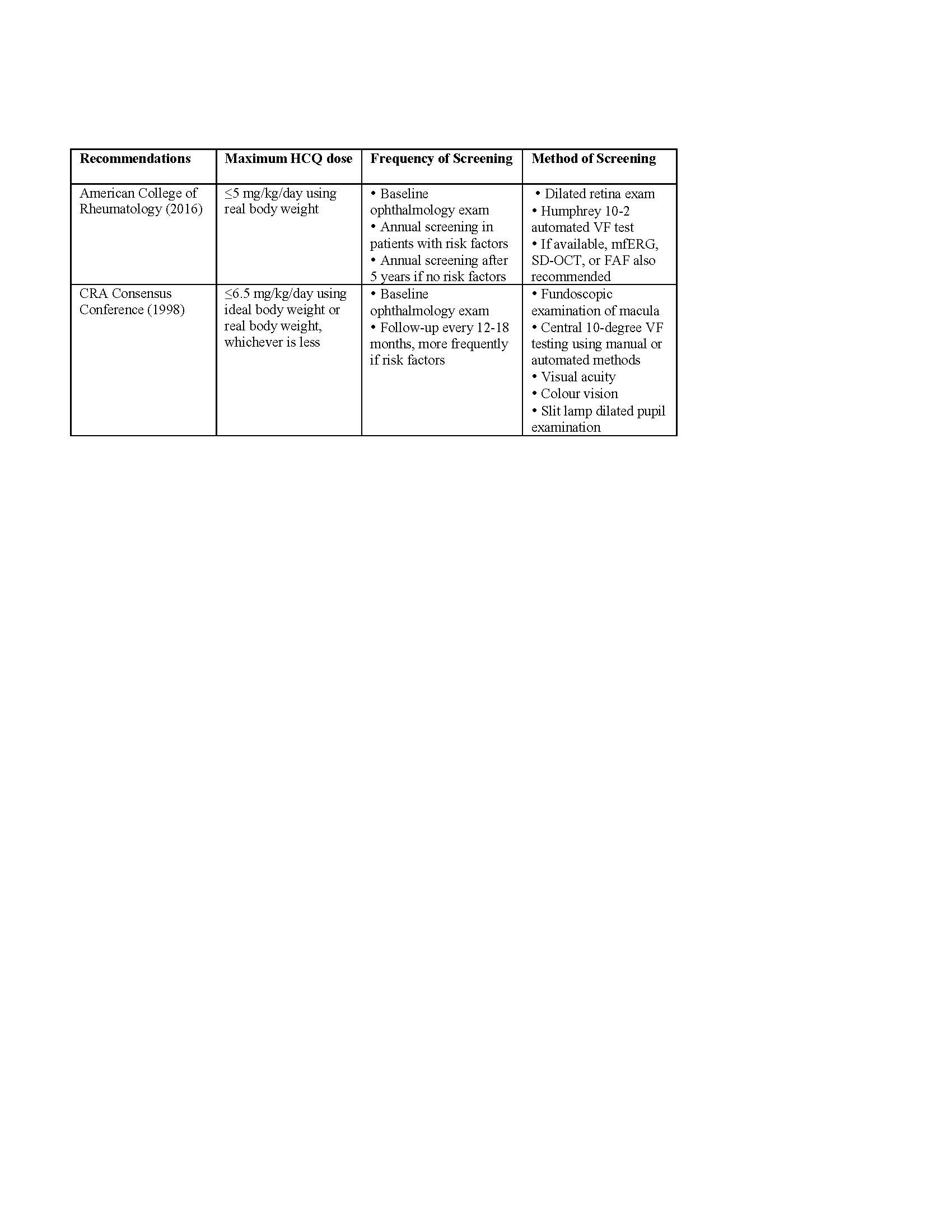
Table 2. Recommendations from Rheumatology Associations
HCQ: Hydroxychloroquine; VF: Visual Fields; SD-OCT: Spectral-Domain Optical Coherence Tomography; mfERG: Multifocal Electroretinography; FAF: Fundus Autofluorescence
To cite this abstract in AMA style:
Cramarossa G, Pope J, Turk M. Guidelines on Prescribing and Monitoring Antimalarials in Rheumatic Diseases: A Systematic Review [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/guidelines-on-prescribing-and-monitoring-antimalarials-in-rheumatic-diseases-a-systematic-review/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/guidelines-on-prescribing-and-monitoring-antimalarials-in-rheumatic-diseases-a-systematic-review/
