Session Information
Session Type: ACR Late-breaking Abstract Session
Session Time: 9:00AM-11:00AM
Background/Purpose: Since
the 2009 GRAPPA treatment recommendations were published, therapeutic options
and management strategies for psoriatic arthritis (PsA) have advanced considerably.
We updated and improved these recommendations through development of
overarching management principles and evidence based guidance for treatment of
the different manifestations of PsA including associated comorbidities.
Methods: GRAPPA
rheumatologists, dermatologists, and patient research partners (PRPs) drafted
overarching principles for the management of adult PsA patients by consensus. We
updated systematic literature reviews based on data publicly available through
November 2014. Six separate literature reviews were performed covering
treatment for the key clinical manifestations of PsA (arthritis, spondylitis,
enthesitis, dactylitis, skin, and nail disease), and we reviewed a new topic
(PsA related comorbidities). Evidence was assessed, and the GRADE system was
applied to generate strong or conditional recommendations for therapies within
the domain groups and for the management of comorbidities. These
recommendations were then incorporated into an overall treatment schema. Consensus
for the overarching principles and treatment recommendations was obtained among
GRAPPA members with an online questionnaire.
Results: GRAPPA members
voted on six overarching principles developed through multiple iterations with significant
agreement among both health care professionals (HCPs; n=135, agreement
83.7-92.6% for individual principles) and PRPs (n=10, agreement 80-100%). Evidence
was assessed from the published literature reviews and formally evaluated with
the GRADE system to provide treatment recommendations (table 1). Based on the evidence,
individual groups developed treatment recommendations which were incorporated
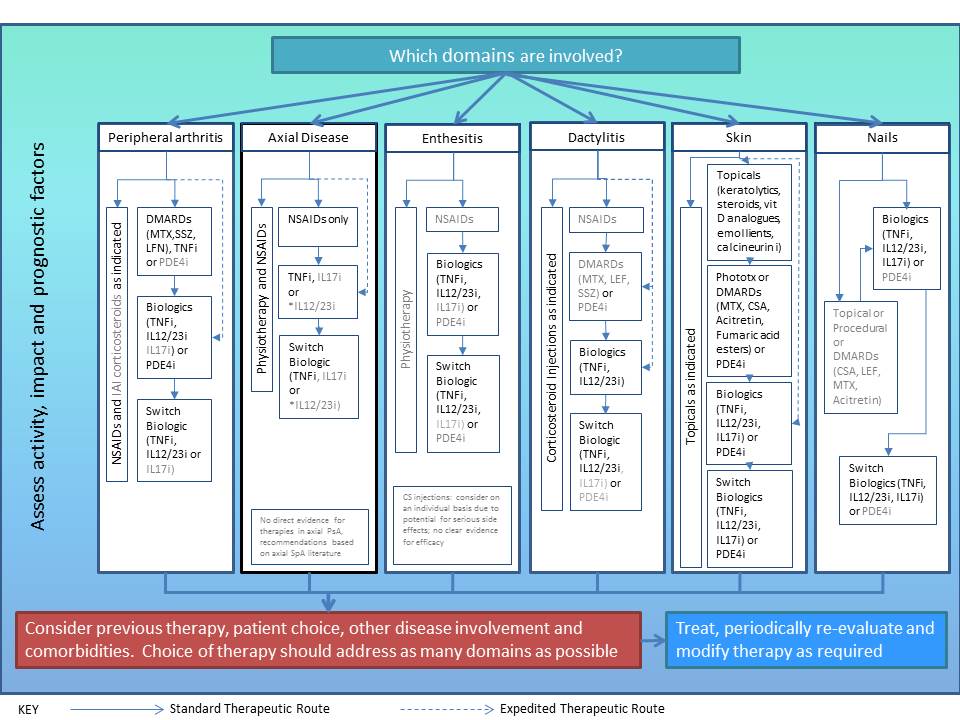
into an overall schema including principles for management of arthritis,
spondylitis, enthesitis, dactylitis, skin, and nail disease, and comorbidities in
PsA (figure 1). Consensus amongst the GRAPPA members was 87.2% agreement
(n=176) for the treatment recommendations and 87.9% (n=176) for the schema.
Conclusion: Based on the
results of the literature review and GRADE assessment of the evidence, we developed
updated comprehensive treatment recommendations for the key manifestations of
PsA, including related comorbidities. These recommendations are supplemented
by overarching principles developed by consensus of GRAPPA members
(rheumatologists, dermatologists, other HCPs, and PRPs).
|
Indication |
Recommended Strong |
Recommended Weak |
Not recommended Strong |
No recommendations as lack of evidence |
|
Peripheral Arthritis DMARD Naïve |
DMARDs (MTX, SSZ, LEF), TNFi |
NSAIDs, oral CS, IA CS, PDE4i |
|
IL12/23i, IL17i |
|
Peripheral Arthritis DMARD Inadequately Responsive |
TNFi, ustekinumab, PDE4i |
NSAIDs, oral CS, IA CS, IL17i |
|
|
|
Peripheral Arthritis Biologic Inadequately Responsive |
TNFi |
NSAIDs, oral CS, IA CS, IL12/23i, IL17i, PDE4i |
|
|
|
Axial PsA, Biologic Naïve (based on AS literature) |
NSAIDs, Physiotherapy, simple analgesia, TNFi |
IL17i, CS SIJ injections, bisphosphonates (IL12/23i) |
DMARDs, IL6i, CD20i |
|
|
Axial PsA, Biologic Inadequately Responsive (based on AS literature) |
NSAIDs, Physiotherapy, simple analgesia |
TNFi, IL12/23i, IL17i |
|
|
|
Enthesitis |
TNFi, IL12/23i, |
NSAIDs, physiotherapy, CS injections (with extreme caution since injecting CS in weight-bearing entheseal sites can lead to rupture of entheses), PDE4i, IL17i |
|
DMARDs |
|
Dactylitis |
TNFi (INF, ADM, GOL, CZP) |
CS injections, DMARDs (MTX, LEF, SSZ), TNFi (ETN), IL12/23i, IL17i (SEC), PDE4i |
|
|
|
Psoriasis (plaque) |
Topical therapies, phototherapy, DMARDs (MTX, LEF, CyA), TNFi, IL12/23i, IL17i, PDE4i |
|
|
|
|
Nail psoriasis |
TNFi, IL12/23i |
Topical therapies, procedural therapies, DMARDs (CyA, LEF, Acitretin, MTX), IL17i, PDE4i |
|
|
|
Italicized text identifies conditional recommendations for drugs without current regulatory approvals or where recommendations are based on abstract data only. Italicized text in brackets identifies a conditional recommendation based only on abstract data from a small open-label proof-of-concept trial. ADM = adalimumab, AS = ankylosing spondylitis, CD20i = CD20 inhibitor, CS = corticosteroids, CyA = cyclosporin, CZP = certolizumab, DMARDs = disease modifying anti-rheumatic drugs, GOL = golimumab, IA = intra‑articular, IL6i = interleukin 6 inhibitor, IL17i = interleukin 17 inhibitor, IL12/23i = interleukin 12/23 inhibitor, INF = infliximab, LEF = leflunomide, MTX = methotrexate, NSAIDs = nonsteroidal anti-inflammatory drugs, PDE4i = phosphodiesterase 4 inhibitor (apremilast), SEC = secukinumab, SIJ = sacroiliac injections, SSZ = sulfasalazine, TNFi = tumor necrosis factor inhibitor |
||||
To cite this abstract in AMA style:
Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, Bautista-Molano W, Boehncke WH, Campbell W, Cauli A, Espinoza L, FitzGerald O, Gladman DD, Gottlieb AB, Helliwell PS, Husni ME, Love T, Lubrano E, McHugh NJ, Nash P, Ogdie-Beatty A, Orbai AM, Parkinson A, O'Sullivan D, Rosen CF, Schwartzman S, Siegel E, Toloza S, Tuong W, Ritchlin CT. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): Treatment Recommendations for Psoriatic Arthritis 2015 [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-grappa-treatment-recommendations-for-psoriatic-arthritis-2015/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/group-for-research-and-assessment-of-psoriasis-and-psoriatic-arthritis-grappa-treatment-recommendations-for-psoriatic-arthritis-2015/