Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Real world utilization of biologic therapy for inflammatory arthritis often falls short of international best practice and is heavily influenced by affordability and national healthcare financing models. Healthcare in Singapore is delivered through a complex mix of regulated compulsory personal savings, limited hospitalization-focused personal and government aided medical insurance, and means-tested government subsidies for selected clinical indications. We describe our experience in translating current evidence into an applicable framework to guide government funding decisions to facilitate equitable access to biologic therapy.
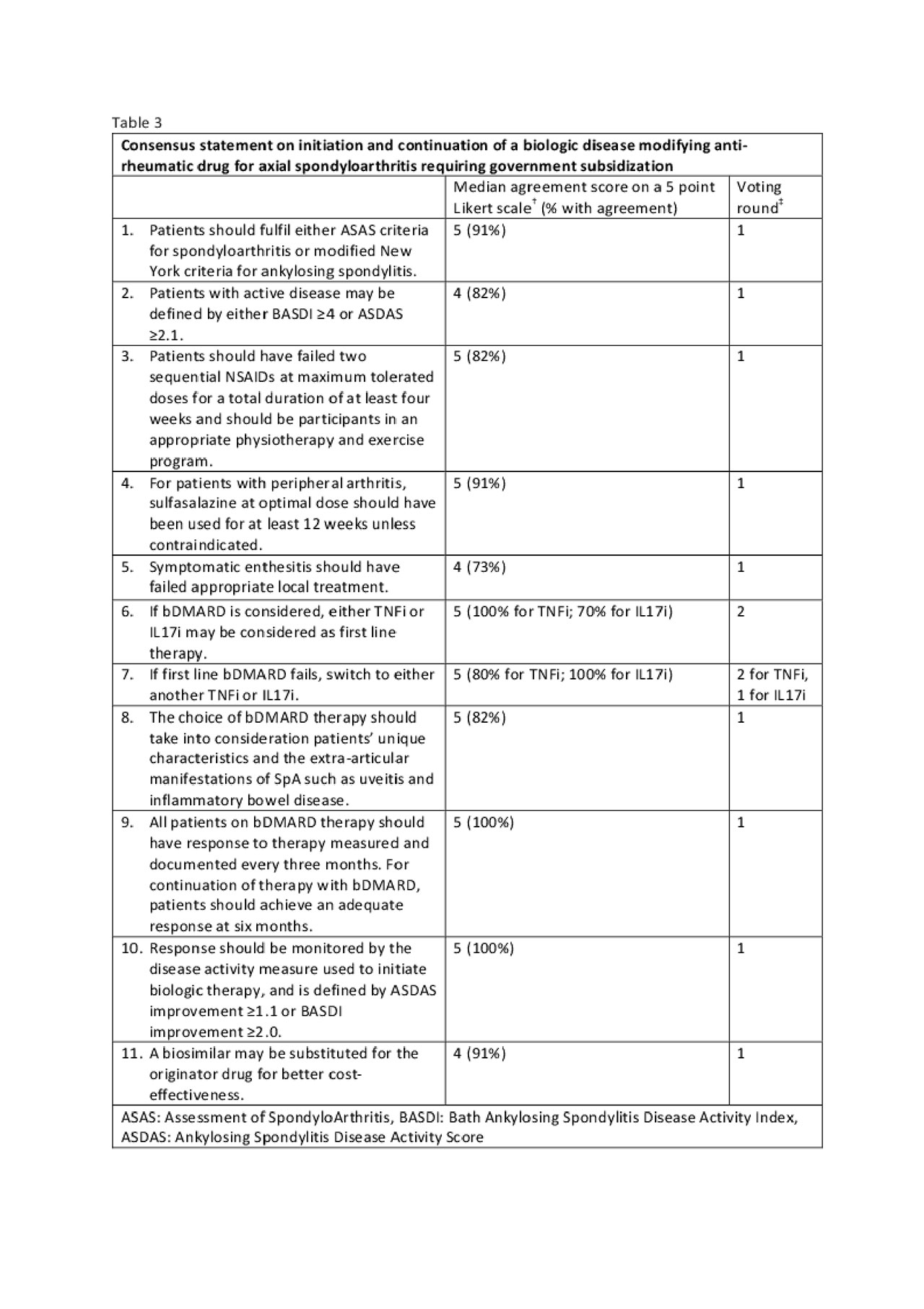
Methods: A core working group (CWG) searched recently published guidelines of biologic agents in rheumatoid arthritis (RA), psoriatic arthritis (PsA) and axial spondyloarthritis (AxSpA) developed for best practice use (9 RA, 5 PsA and 4 AxSpA) or reimbursement (2 RA, 4 PsA and 2 AxSpA). Where appropriate, recent primary literature was reviewed and the evidence synthesized in the form of tables. A modified Delphi approach was used and statements were drafted for rating by an expert task force panel (TFP) comprising rheumatologists practicing in the public and private sectors across Singapore. The TFP independently rated 3 separate sets of draft statements for RA, PsA and AxSpA after consideration of the synthesized evidence. Consensus was reached if there was at least 70% agreement (score of 4 or 5 on a five-point Likert scale). Statements not reaching consensus were discussed in a face to face meeting, and second round of independent voting was done, after rewording and/or editing statements if necessary. Next, government agencies were engaged to adopt the final statements to guide government funding. The guidelines will be disseminated to the relevant pharmaceutical companies to allow them to make informed decisions on drug pricing to be considered favourably in the government tender.
Results: Three sets of consensus statements were derived, pertaining to initiation, choice and continuation of biologic therapy for active RA, PsA or AxSpA (tables 1, 2, 3 respectively). These form an easily applicable framework to be used by practicing physicians and funding authorities.
Conclusion: We describe our experience in formulating evidence-based guidelines within the real-world practice which is heavily influenced by treatment affordability. Lessons learnt in engaging all relevant stakeholders may be applicable to guide nascent subsidization programs in healthcare economies worldwide.
To cite this abstract in AMA style:
Chua K, Leong W, Phang K, Dissanayake S, Cheung P, Fong W, Leong K, Leung Y, Lim A, Lui N, Manghani M, Santosa A, Sriranganathan M, Suresh E, Tan T, Teng G, Lahiri M. Government Subsidization of Biologic Therapy for Inflammatory Arthritis in a Co-Funded Healthcare Model: A Singapore Experience [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/government-subsidization-of-biologic-therapy-for-inflammatory-arthritis-in-a-co-funded-healthcare-model-a-singapore-experience/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/government-subsidization-of-biologic-therapy-for-inflammatory-arthritis-in-a-co-funded-healthcare-model-a-singapore-experience/