Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Inflammatory activity, as well as traditional cardiovascular risk factors, have been suggested to underlie the increased risk of coronary disease in patients with rheumatoid arthritis (RA). Treatment with tumour necrosis factor inhibitors (TNFi) may often effectively reduce disease activity in RA patients. The objective of the study was to evaluate whether level of response to TNFi in RA are associated with the risk of developing an acute coronary syndrome (ACS).
Methods:
In the Swedish Biologics Register we identified a cohort of patients, with RA and no previous IHD, who had been on TNFi treatment > 2 months 2001-2010 (n=7,361). For each patient 5 matched referents were randomly selected from the Population Register. Information on inflammatory activity (ESR, CRP, DAS28, CDAI) for the patients was extracted from the register at start and from a visit 5 (±3) months after start of therapy. Data on the primary exposure, EULAR response at 5 months, was available in 4,931 (67%) individuals, mean age 57.1 years, 75.9% women. Covariates were extracted from the Patient register (prevalent hypertension, diabetes, chronic pulmonary disease, infection, and atherosclerotic disease, joint surgery, disease duration) and Statistics Sweden (educational level and work disability). The outcome, incident ACS, was defined as a primary discharge diagnosis of myocardial infarction or unstable angina, or myocardial infarction as the underlying cause of death. Incidence rates were calculated and Cox Proportional Hazard Regression models were utilized for risk estimations.
Results:
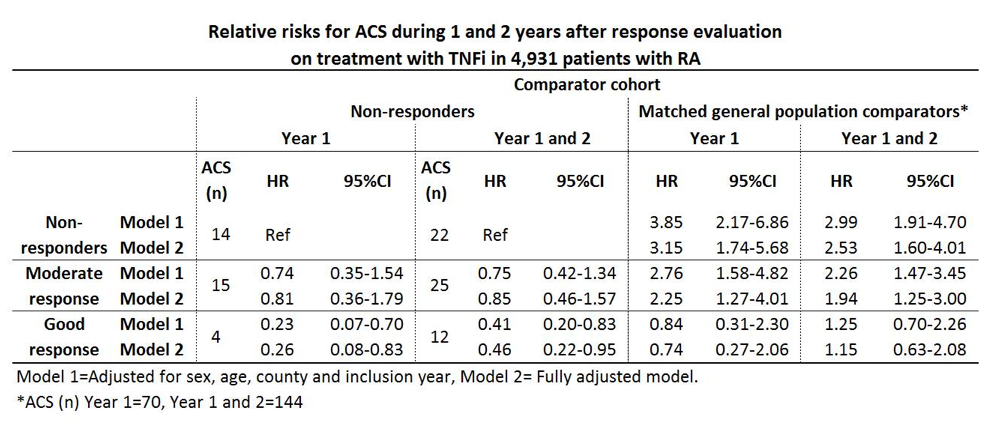
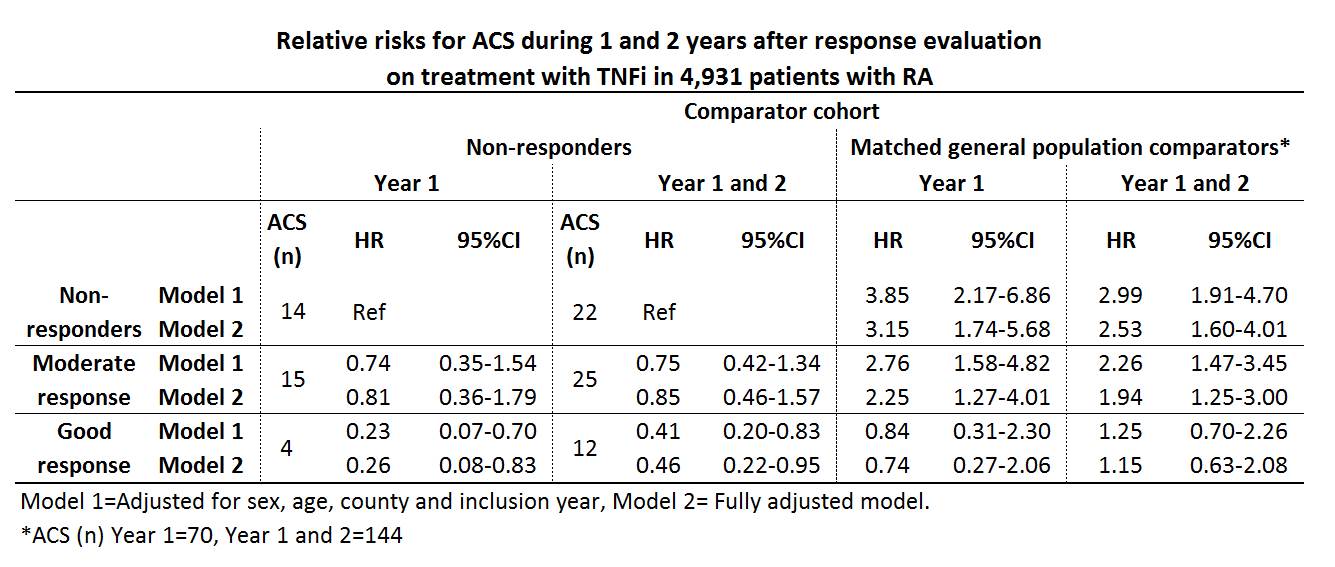
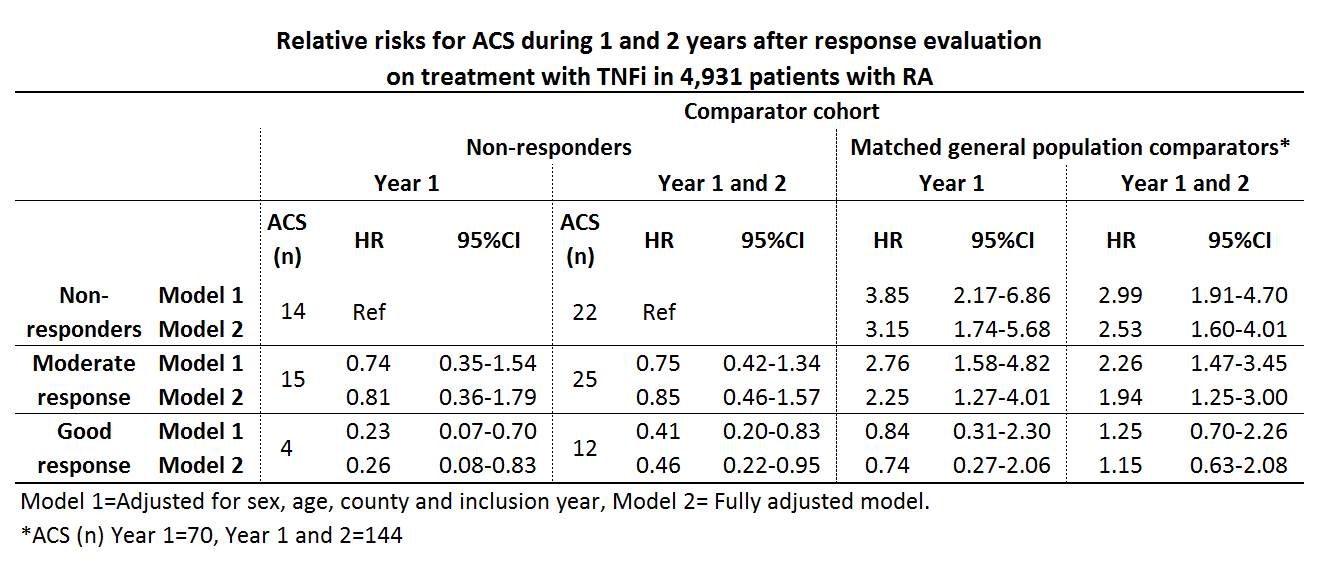
A good EULAR response was observed in 37.8% (n=1866), a moderate response in 37.0% (n=1824) and no response in 25.2% (n=1241) of the patients. During the 1st year after the response evaluation 33 ACS were observed among the patients, and during year 1 and 2 after exposure 59 ACS were observed, resulting in crude incidence rates of (IR (95%CI)) 7.18 (5.10-10.10) and 6.90 (5.34-8.90) per 1,000 person-years, respectively. Among the general population comparators the corresponding IRs were 3.05 (2.42-3.86) and 3.37 (2.86-3.97). Relative risks for ACS among the response classes compared with non-responders and general population referents are presented in table.
Fully adjusted relative risks the year after evaluations for the secondary exposures were; ESR<20 vs. ESR³20 HR 0.34 (0.16-0.74), CRP²10 vs. CRP>10 HR 0.77 (0.38-1.54), DAS28 remission vs. non-remission HR 0.21 (0.05-0.88) and CDAI remission vs. non-remission HR 0.44 (0.06-3.27).
Conclusion:
Good EULAR response after 5 months of treatment with TNFi in RA patients was associated with a significantly decreased risk of ACS, as was ESR<20 and DAS28 remission. In patients with good response on therapy no significant increase in the risk of ACS was detectable in comparison with the risk in general population during the 2 years after the evaluation.
Disclosure:
L. Ljung,
Bristol Myers Squibb,
5;
L. T. H. Jacobsson,
Pfizer ,
2,
Pfizer ,
5,
UCB,
5,
Abbvie ,
5;
S. M. Rantapää-Dahlqvist,
None;
J. Askling,
Pfizer Inc,
2;
T. ARTIS Study Group,
Merck, BMS, Pfizer, Abbott Laboratories, SOBI, UCB, and Roche,
9.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/good-response-on-tumour-necrosis-factor-inhibitors-are-associated-with-a-decreased-risk-of-acute-coronary-syndromes-in-patients-with-rheumatoid-arthritis/