Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy of Golimumab (GLM) treatment in rheumatoid arthritis (RA) has been widely documented. The aim of this study was to analize the long-term retention of GLM and to identify independent predictors of drug retention in patients with AR.
Methods: Prospective monocentric cohort of RA patients treated with GLM according with clinical practice. Study was approved by local Ethics Committee. Demographic and clinical variables were analyzed with Cox proportional hazard regression model.
Results: 61 patients were included. The baseline characteristics of the patients are shown in Table 1. Mean follow-up time 26.8 months (SD 26.1). Mean survival time was 46,3 months (95% CI: 35.6-57.1) Age, gender, DAS28 at baseline, rheumatoid factor (RF), antibodies anti–cyclic citrullinated peptide (anti-CCP), concomitant DMARD and previous treatment with biological therapy were considered in the univariate analysis.
Table 1. Baseline demographic and clinical characteristics of the patients.
|
Age -mean (SD)- years |
55.1 (14.1) |
|
Female gender % |
85.2% |
|
Mean evolution time (SD) years |
10.2 (8.1) |
|
SCJ -mean-(SD) |
4.1 (3.6) |
|
DAS28 -mean-(SD) |
4.7 (1.4) |
|
RF+ % |
70% |
|
Anti-CCP + % |
78% |
|
Concomitant DMARD % |
74,6% |
|
Biological therapy naïve % |
53.3% |
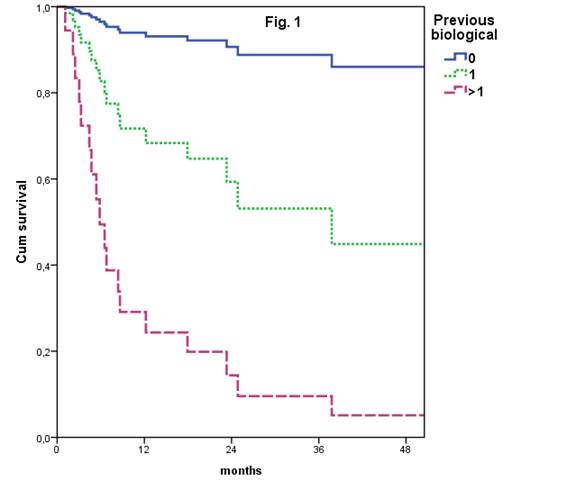
Upon Cox regression analysis, concomitant DMARD [HR 2.6 (95% CI 1.2-5.8)] and use of GLM as first biological [HR 6.3 (95% CI 2.5-15.8)] were predictors of better GLM retention rate. When analyzing the number of previous biologicals, GLM retention rate was not statistically different as first or second biologic [HR 5.3 (95% CI: 0.8-32.4)]. GLM retention rate was significantly worst when used as third or fourth biological [HR 19.8 (95% CI 4.3-89.9)]. Figure 1. 27/61 (44.3%) patients discontinued GLM, 18/27 (66.7%) due to lack of efficacy, 8/27 (29.6%) due to adverse event and 1/27 (3.7%) for other reasons.
Conclusion: Real life long term Golimumab retention rate was good. Golimumab survival time was better when used as first or second biological and with concomitant DMARD.
To cite this abstract in AMA style:
Serrano B, Gonzalez CM, González R, Martínez-Barrio J, Ovalles-Bonilla JG, Nieto JC, Janta I, Valor L, López Longo FJ, Monteagudo I. Golimumab Retention Rate in Patients with Rheumatoid Arthritis. Predictors of Long-Term Retention [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/golimumab-retention-rate-in-patients-with-rheumatoid-arthritis-predictors-of-long-term-retention/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/golimumab-retention-rate-in-patients-with-rheumatoid-arthritis-predictors-of-long-term-retention/