Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Golimumab is a monthly self-injected anti-tumor necrosis factor alpha therapy for patients with rheumatoid arthritis (RA), ankylosing spondylitis (AS), and psoriatic arthritis (PsA). It was approved by Health Canada in April 2009 and this is the first Canadian report of golimumab’s utilization in a real-world setting. The purpose of this project was to assess the utilization patterns of rheumatoid arthritis patients who received golimumab (GLM), etanercept (ETA) or adalimumab (ADA) in a large private drug plans database.
Methods: RA patients initiating therapy with GLM, ETA or ADA between January 1, 2009 and June 30, 2010 were selected from the IMS Brogan Canadian private drug plans database. Patients were included if they had ≥ 3 claims for GLM, ETA or ADA and were naïve to biologics prior to initiation of treatment. The 6-month period preceding the first GLM, ETA or ADA claim (index date) for each patient was used as a baseline to ensure that the patient were naïve to biologics. Patients were tracked for a maximum of 12 months after their index date to assess dosing patterns. The average dose in mg/week and the % of discontinuation (defined as the gap between 2 consecutive claims exceeding 60 days or switching to another drug) were evaluated.
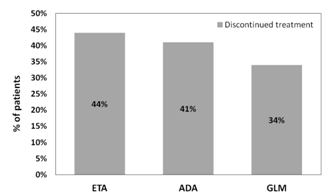
Results: A total of 146, 1436 and 1171 RA patients receiving at least three prescriptions of GLM, ETA or ADA respectively were identified. Within the period of analysis, patients treated with ETA had a numerically higher discontinuation rate (44%) followed by ADA patients (41%) whereas patients treated with GLM had the lowest discontinuation rate (34%). The average dose of GLM, ETA or ADA were respectively 51.6 mg/month, 46.7 mg/week and 42 mg/q2weeks in accordance with their respective label in Canada for RA.
Figure 1. Treatment discontinuation rate within a period of 12 month following initiation (Bionaïve at initiation)
Conclusion: Results of these descriptive claims analyses show that in a real-world setting, the use of golimumab in RA patient’s naïve to biologics is in accordance with the approved dose of 50 mg/month and that a numerically higher drug survival rate is observed for GLM (76%) compared to ETA and ADA 1 year after initiation of treatment. This is the first time that drug utilization patterns of GLM in comparison to ETA and ADA is reported in a real-world setting. Further research is needed to compare the relationship between drug utilization patterns on healthcare resource utilization and outcomes.
Disclosure:
H. Khalil,
Janssen Canada Inc,
3;
A. Tahami,
Janssen Canada Inc.,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/golimumab-drug-utilization-patterns-in-canada-higher-retention-rate-in-golimumab-treated-rheumatoid-arthritis-patients-compared-to-etanercept-and-adalimumab/