Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: A previous retrospective study suggested superiority of glucocorticoids (GCs) plus rituximab (RTX) compared to GCs alone to induce complete clinical response in non-infectious cryoglobulinemia vasculitis (CryoVas). However, GCs plus RTX regimen was associated with severe infections, whereas death rates did not differ between therapeutic regimens. The ESBAM trial (NCT02556866) aimed to evaluate the efficacy and safety of RTX in combination with GCs for the treatment of non-infectious active mixed CryoVas.
Methods: We conducted a multicenter, randomized, double-blind, superiority trial of RTX as compared with placebo for remission induction in non-infectious active mixed CryoVas. To be included, patients had to have an active CryoVas defined by positive serum cryoglobulin and an active vasculitis, and to be treatment-naive or relapsing patients. Patients were randomized to receive prednisone plus RTX administered by intravenous infusion at 375 mg/m2 at day (D) 1, D8, D15 and D22, or prednisone plus placebo administered following the same schedule.GCs were tapered off. The primary endpoint was the remission of mixed CryoVas without the use of prednisone at week (W) 24. This study was initially planned to include 79 patients per group.
Results: Between July 2015 and July 2017, 15 patients were enrolled in the ESBAM trial and 14 were randomized (one patient died before randomization): 5 to receive RTX and 9 to the placebo-group. Their median age was 62 [45;71] years, 73% were women, and 73% relapsing-patients. Main CryoVas manifestations included: purpura (71%), neuropathy (43%), arthralgias (36%) and glomerulonephritis (29%). Patients’ characteristics were comparable between groups.
At W24, 4/5 (80%) had an inactive vasculitis in the RTX-group compared to 6/8 (75%) in the placebo-group. A remission with no prednisone was achieved in 1/5 (20%) patient the RTX-group vs. 0/8 in the placebo-group. Cumulative doses of GCs at W24 and W48 were comparable in the two groups.
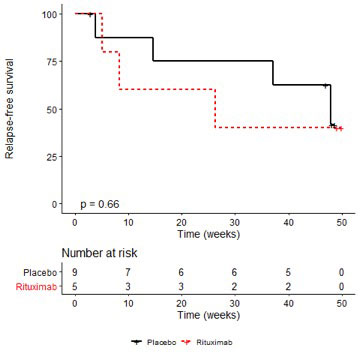
During the 48-week follow-up, 7 vasculitis relapses occurred, i.e. 3 in the RTX-group vs. 4 in the placebo-group.Relapse-free survival rates at W48 were 40.0% [13.7–100%] in the RTX-group vs. 41.7% [15.9–100%] in the placebo-group (Figure). The SF-36 physical health summary significantly improved in the RTX-group compared to placebo-group, especially at W36 and W48 (P=0.02 and P=0.04). Cryoglobulinemia remained positive in both groups for the majority of patients, and evolution of serum C4 fraction was comparable between groups during the 48 weeks. Twenty severe adverse events were recorded, 6 in the RTX-group and 20 in the placebo-group, including 1 patient in the RTX-group and 2 in the placebo-grouphaving severe infections. No patient died after randomization.
Conclusion: Combination of glucocorticoids plus rituximab and glucocorticoids plus placebo seemed to induce similar rates of remission at week 24 in non-infectious active mixed cryoglobulinemia vasculitis.
To cite this abstract in AMA style:
Terrier B, London J, Bonnet F, Cerutti D, Costedoat-Chalumeau N, Diot E, Ferfar Y, Hummel A, Kaplanski G, Marie I, Quémeneur T, Rullier P, Senet P, Le Gouellec N, Lejeune J, Saadoun D, Cacoub P. Glucocorticoids Plus Rituximab versus Glucocorticoids Plus Placebo in Non-infectious Active Mixed Cryoglobulinemia Vasculitis: Results of a Placebo-Controlled Randomized Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/glucocorticoids-plus-rituximab-versus-glucocorticoids-plus-placebo-in-non-infectious-active-mixed-cryoglobulinemia-vasculitis-results-of-a-placebo-controlled-randomized-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoids-plus-rituximab-versus-glucocorticoids-plus-placebo-in-non-infectious-active-mixed-cryoglobulinemia-vasculitis-results-of-a-placebo-controlled-randomized-trial/