Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoid (GC) use and adverse effects (AEs) are prevalent in rheumatic diseases, yet there is no standardized patient-reported outcome measure to assess benefit and risk. This study aims to determine the AEs related to GCs in two cohorts of GC users and determine the benefits and risks from the patient perspective. A secondary aim was to compare AEs amongst RA patients both exposed and unexposed to GCs.
Methods: Participants in cohort 1 attended an Australian tertiary rheumatology clinic with various rheumatic diseases and were taking an oral GC currently or within the past 12 months. Cohort 2 was from the Hospital for Special Surgery RA database (all met ACR/EULAR RA criteria) and included both GC users and non-users. The survey included a checklist of 19 known AEs and an open-ended question about presence of ‘other GC side effects’. The median number of AEs experienced by each patient was compared between cohorts using poisson regression. All participants were asked to rate the three ‘worst’ AEs. Participants exposed to GCs were asked to indicate whether GC therapy helped ‘a lot’, ‘a little’, ‘not sure’ or ‘not at all’ and the ordinal trend between groups was compared using the Cochran Armitage exact test. GC-users were also asked whether the AEs they experienced were worse than the benefits of treatment (Yes/No/Not sure), and analyzed by chi-square.
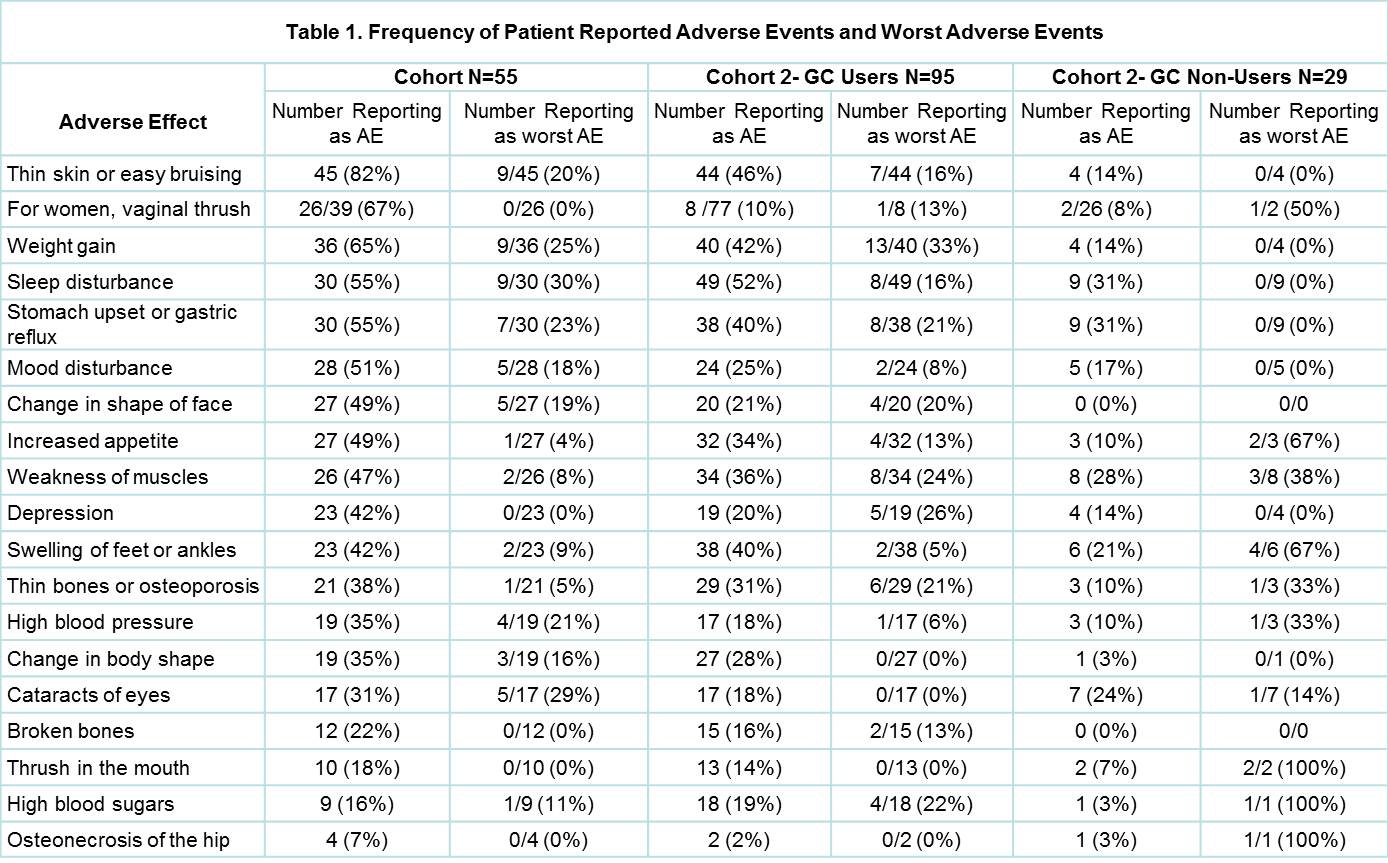
Results: There were 55 participants from cohort 1 (71% female, median age 68, range 33-89yrs) and 124 from cohort 2 (83% female, median age 63, range 27-82). The disease range amongst cohort 1 was broad, with CTDs (14/55), RA (4/55), PMR (14/55) and GCA (5/55) most common. Amongst Cohort 2, 95 (77%) had ever used GCs (GC-users) and 29 (23%) were GC non-users. The median number of AEs was higher in cohort 1(7.7, 95% CI 7.0-8.5) compared to GC users in cohort 2, (5.3, 95% CI4.9-5.8), and both were higher than GC non-users (2.6, 95%CI 2.1-3.3). All patients in cohort 1 reported at least one GC AE compared to 86% of GC-users in Cohort 2 (p=0.002). The frequency of patient reported AEs and worst AEs are shown in Table 1. In both GC use cohorts, the majority (73%/62%) felt GCs helped their disease ‘a lot’, 11%/21% felt they helped ‘a little’, 9%/8% were ‘not sure’ and 2%/8% felt GCs did not help at all, with no difference between groups (ordinal p=1.0). Most participants in cohort 1(55%) and 2 (64%) reported that the benefits of treatment were greater than the AEs (p=0.67).
Conclusion: Apart from weight gain, AEs that are important from the patient perspective are poorly captured using current measures, and patients with different diagnoses may rate GC AEs and benefits differently. These hypothesis generating surveys reveal the need for further study and to develop a patient reported outcome measure for GC AEs and benefits so patients with rheumatic diseases can participate in informed treatment choices.
To cite this abstract in AMA style:
Black R, Goodman SM, Ruediger C, Lester S, Mackie S, Hill C. Glucocorticoid Adverse Effects – the Patient Perspective [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/glucocorticoid-adverse-effects-the-patient-perspective/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/glucocorticoid-adverse-effects-the-patient-perspective/