Session Information
Date: Sunday, October 26, 2025
Title: (0731–0764) Vasculitis – Non-ANCA-Associated & Related Disorders Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: The optimal duration of tocilizumab (TCZ) treatment for giant cell arteritis (GCA) is unclear. Observational studies have shown that 50-60% of patients relapse after receiving TCZ for ≥12 months in combination with ≥6-10 months of glucocorticoids (GC) [1]. However, there are no data on outcomes after treatment discontinuation in patients receiving TCZ with shorter GC tapers. We investigated the relapse rate of GCA patients after treatment with 2 months of prednisone and 12 months of TCZ.
Methods: The electronic medical records of patients with GCA who completed a pilot clinical trial of TCZ in combination with a short prednisone taper (11/2018-12/2021) [1] were retrospectively reviewed. During the clinical trial, in the absence of relapse, patients weaned off prednisone within 2 months and discontinued TCZ at month 12. The primary endpoint of the analysis was relapse after month 12. Relapse was defined as the recurrence of GCA signs or symptoms that required treatment, regardless of the ESR and CRP values. Time to relapse and features of relapse were assessed. Factors potentially associated with relapse were assessed in univariate analyses and multivariate logistic regression.
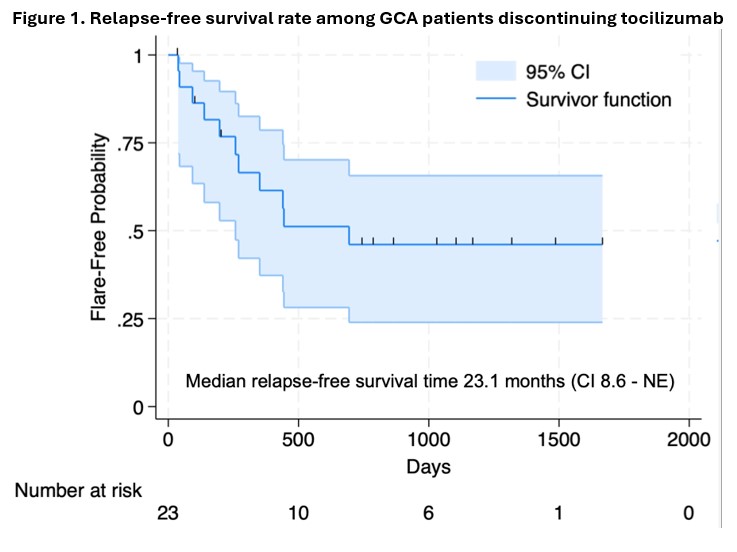
Results: The results of the clinical trial were published elsewhere [1]. In brief, of 30 patients receiving 12 months of TCZ and 2 months of prednisone, 23 (77.7%) maintained disease remission through month 12 and 7 (23.3%) relapsed before month 12 [1]. The 23 patients that maintained remission received a median (IQR) cumulative prednisone dose of 962.5 (682.5, 1610) mg during the trial and were followed for a median (IQR) period of 36 (22, 44) months after the trial ended. Other baseline characteristics are shown in Table 1. During follow-up, 11 (47.8%) patients relapsed following TCZ discontinuation (Table 2). The median relapse-free survival time was 23.1 (95% CI 8.6-NE) months (Figure 1). At relapse, 4 (36.4%) patients had cranial symptoms and 7 (63.6%) had polymyalgia rheumatica symptoms (Table 2). One (9.1%) patient had biopsy-proven aortitis despite being asymptomatic. No patients reported visual symptoms at the time of relapse. The median (IQR) ESR (mm/hour) and CRP (mg/liter) values at relapse were 39 (25, 43.75) and 10.5 (5.6, 24.73), respectively. Of all relapsing patients, 10 (90.9%) received prednisone. In addition, 6 (54.5%) patients re-initiated TCZ and 2 (18.2%) patients received upadacitinib (Table 2). All patients regained control of their symptoms with treatment. Sex, whether GCA was newly diagnosed at the onset of the clinical trial, cumulative prednisone dose in the year preceding baseline and disease duration did not predict relapse after TCZ withdrawal.
Conclusion: Nearly 50% of patients relapsed after treatment with 12 months of TCZ and 2 months of prednisone. The observed post-treatment relapse rate was similar to that reported in patients receiving TCZ in combination with longer GC tapers, suggesting that the amount and duration of GC treatment does not have a significant effect on the risk of relapse once TCZ is discontinued. Clinical trials to determine the optimal duration of TCZ treatment and assess different TCZ weaning strategies are needed. 1. Unizony et al. Lancet Rheumatol 2023
 Values are n (%) for categorical variables and median (IQR) for continuous variables. * At the time of onset of the clinical trial. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Values are n (%) for categorical variables and median (IQR) for continuous variables. * At the time of onset of the clinical trial. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
.jpg) Values are n (%) for categorical variables and median (IQR) for continuous variables. Fisher’s exact and Wilcoxon rank-sum tests used for univariable comparison of categorical and continuous variables, respectively. A logistic regression model including the variables tested in univariable comparisons did not show predictors independently associated with relapse. * Biopsy-proven aortitis. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
Values are n (%) for categorical variables and median (IQR) for continuous variables. Fisher’s exact and Wilcoxon rank-sum tests used for univariable comparison of categorical and continuous variables, respectively. A logistic regression model including the variables tested in univariable comparisons did not show predictors independently associated with relapse. * Biopsy-proven aortitis. ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
To cite this abstract in AMA style:
O'Dea D, Katz G, Arevalo Molina B, Jarvie A, Matza M, Fernandes A, Stone J, Unizony S. Giant Cell Arteritis Relapse After Treatment with Two Months of Prednisone and 12 Months of Tocilizumab [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/giant-cell-arteritis-relapse-after-treatment-with-two-months-of-prednisone-and-12-months-of-tocilizumab/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/giant-cell-arteritis-relapse-after-treatment-with-two-months-of-prednisone-and-12-months-of-tocilizumab/

.jpg)