Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Disease activity in axial spondyloarthritis (axSpA) is often quantified by the Ankylosing Spondylitis Disease Activity Score (ASDAS), a composite index which combines 4 patient reported outcome (PRO) measures and C-reactive protein (CRP) as an acute phase reactant. ASDAS commonly acts as a target in treat-to-target approaches in axSpA patients. This study aims to identify the impact of gender on ASDAS in newly diagnosed axSpA patients and to determine whether possible differences are PRO- or CRP-driven.
Methods: Patients originate from an observational registry of newly diagnosed axSpA patients (expert opinion). Included patients fulfill the Assessment of SpondyloArthritis international Society (ASAS) classification criteria for axSpA and are anti-TNF naïve prior to inclusion. An extensive patient description was performed at baseline, including PRO and laboratory investigations with CRP (mg/L). ASDAS was calculated for each patient and subdivided in a PRO-component (ASDAS-PRO) and a CRP-component (ASDAS-CRP).
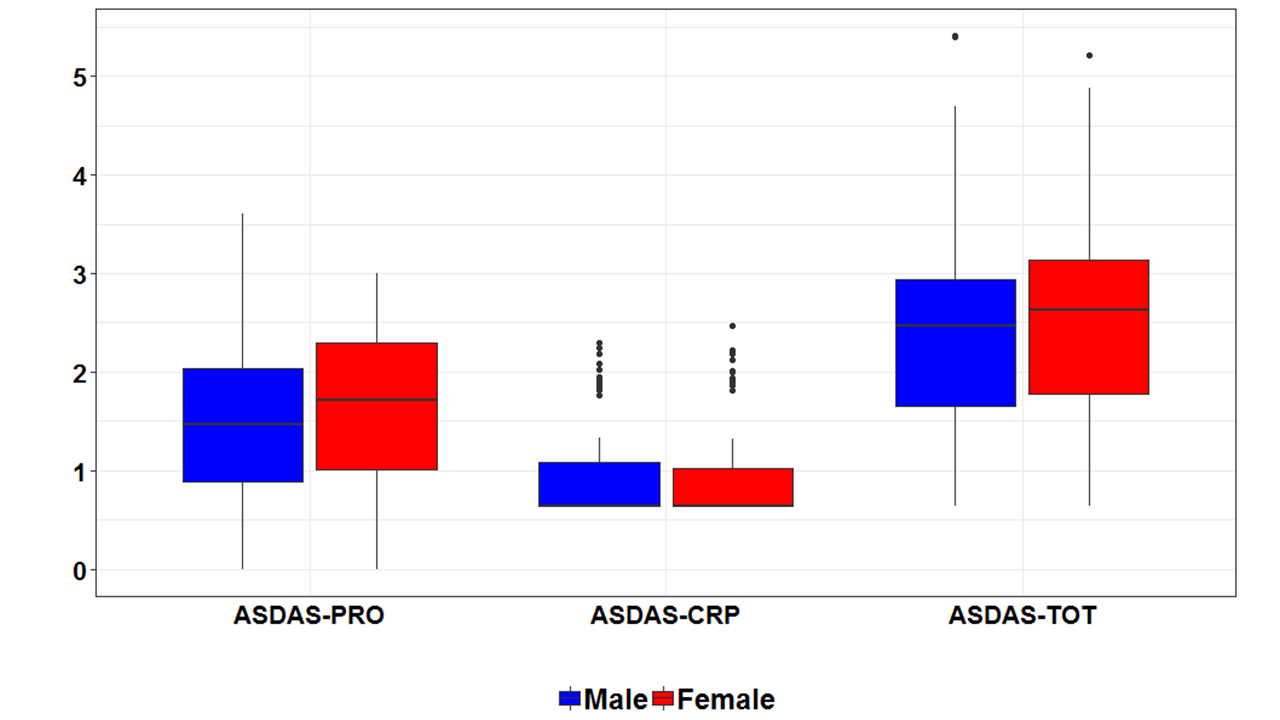
Results: By January 2019, 291 axSpA patients were included (138 male, 153 female). At baseline, ASDAS could be calculated in 262 patients. ASDAS-PRO was significantly higher in women compared to men (1,66 vs. 1,48, p = 0,04), while ASDAS-CRP did not differ significantly (0,90 for both genders, p = 0,96). No significant difference was found between the total ASDAS of women and men (2,54 vs. 2,38, p = 0,09) (Figure 1). When categorizing the score into 4 disease activity states, 60,8% (76/125) of men show high or very high disease activity (ASDAS > 2.1) at baseline compared to 67,2% (92/137) of women (p = 0,35).
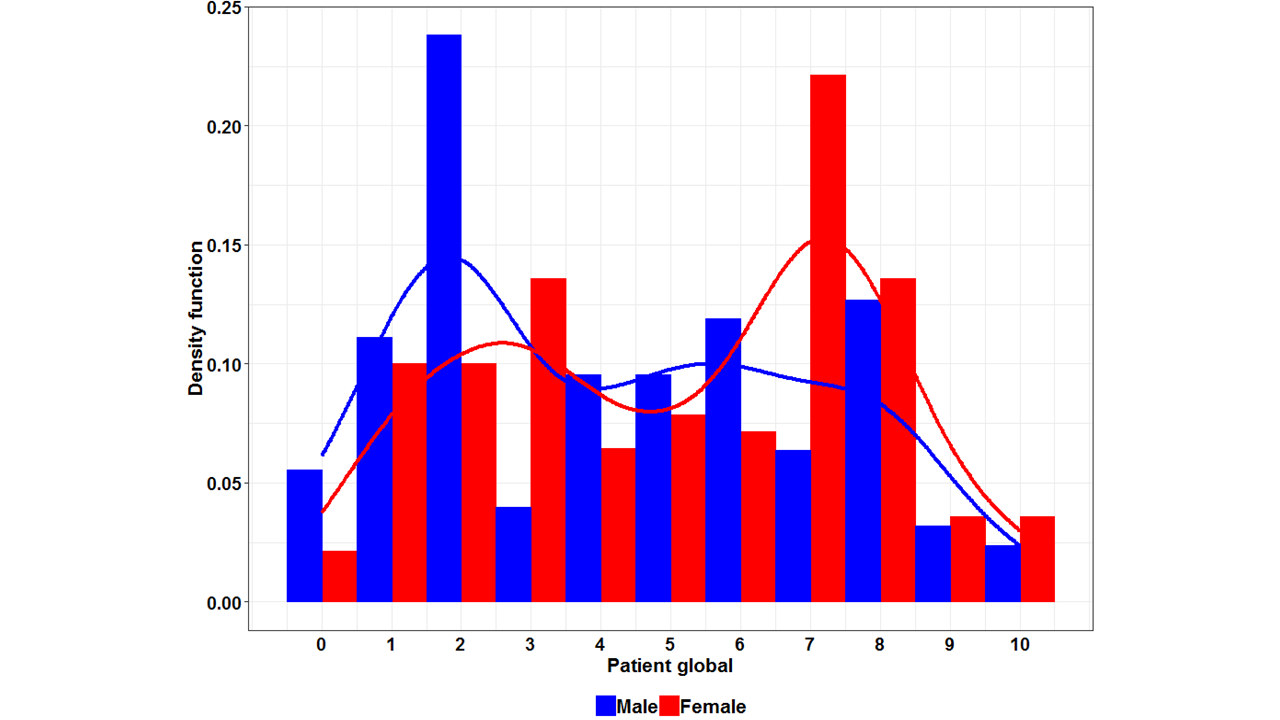
Concerning the 5 ASDAS components, the discrimination between male and female axSpA patients was most pronounced for patient global scores (Figure 2). Men reported a baseline patient global score of 4,3 (95% CI 3,8 – 4,8), compared to a score of 5,1 (95% CI 4,7 – 5,6) in women (p = 0,01).
Conclusion: Female axSpA patients report a significantly higher patient global score compared to men. This contributes to a significantly higher ASDAS-PRO in women. However, neither the ASDAS itself nor the ASDAS disease activity categories are significantly affected by gender contrasts in PRO.
To cite this abstract in AMA style:
De Craemer A, Renson T, Deroo L, de Hooge M, Carron P, Van den Bosch F, Elewaut D. Gender Contrasts in Patient Reported Outcomes Don’t Alter the Disease Activity Score in Axial Spondyloarthritis Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/gender-contrasts-in-patient-reported-outcomes-dont-alter-the-disease-activity-score-in-axial-spondyloarthritis-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/gender-contrasts-in-patient-reported-outcomes-dont-alter-the-disease-activity-score-in-axial-spondyloarthritis-patients/