Session Information
Session Type: Abstract Submissions (ACR)
Further Reduction in Nonvertebral Fracture Rate Is Observed Following 3 Years of Denosumab Treatment: Results With up to 7 Years in the FREEDOM Extension
Background/Purpose: Some antiresorptives reduce nonvertebral fracture incidence in the first 3 years of treatment; however, evidence for further reduction of nonvertebral fractures with prolonged therapy is limited. The effects of denosumab (DMAb) treatment for up to 10 years are being evaluated in the 3-year FREEDOM study and its 7-year extension. Here we compare the nonvertebral fracture rate during the first 3 years of DMAb with that in the following 4 years of treatment.
Methods: During the extension, all subjects received 60 mg DMAb Q6M. Here, long-term subjects received 7 years of DMAb (3 years in FREEDOM followed by 4 years in the extension); subjects from the cross-over group received 4 years of DMAb (3 years of placebo in FREEDOM followed by 4 years of DMAb in the extension). Nonvertebral fracture rates for the first 3 years of DMAb were compared with rates in the 4th year of DMAb in each group separately and combined. For the long-term group only, the nonvertebral fracture rate in the first 3 years of DMAb was also compared with the fracture rate during the subsequent 4 years. Adjusted rate ratios (95% CIs) between observational periods were computed via generalized estimating equation (GEE) Poisson regression.
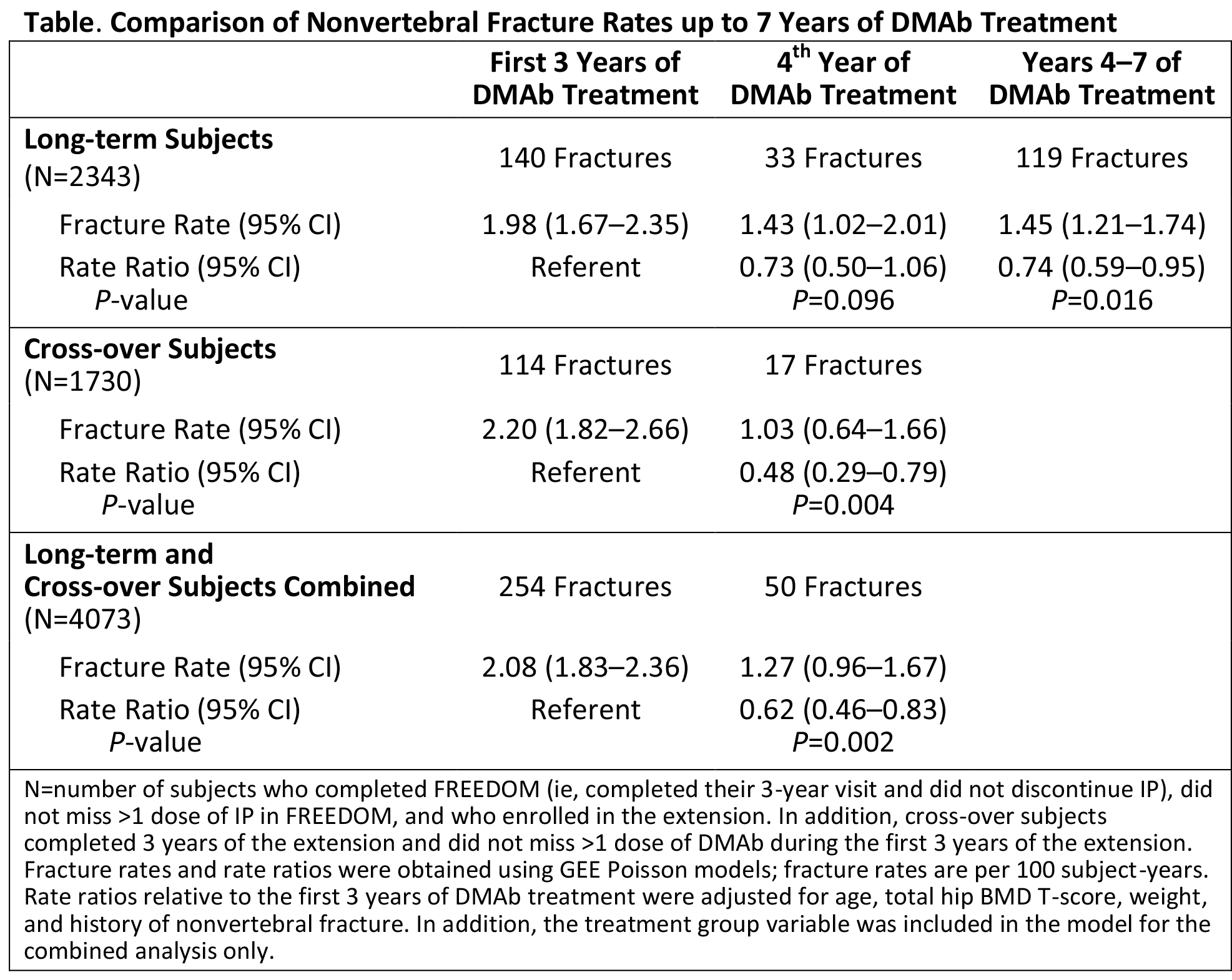
Results: Of 5928 women eligible for the extension, 4550 (77%) enrolled (N=2343 long-term; N=2207 cross‑over). In the long-term group, the nonvertebral fracture rate was 1.98 per 100 subject-years during years 1–3 of DMAb (FREEDOM). This rate decreased during year 4 (extension) to 1.43 (rate ratio=0.73; P=0.096), and the rate remained low at 1.45 during years 4–7 (rate ratio=0.74; P=0.016; Table). Similarly for the cross-over group, the fracture rate was 2.20 during years 1–3 of DMAb (extension) and decreased to 1.03 at year 4 (rate ratio=0.48; P=0.004). Combining the long-term and cross-over groups yielded a fracture rate of 2.08 during the first 3 years of DMAb (FREEDOM or extension) that decreased to 1.27 at year 4 (rate ratio=0.62; P=0.002).
Conclusion: Three years of DMAb treatment significantly reduced the nonvertebral fracture rate compared with placebo. When DMAb was continued for an additional 4 years, the fracture rate remained low and was significantly lower than that in the first 3 years of treatment. Contributing factors that might explain this observation include sustained reduction in bone resorption, continued gains in hip BMD and bone mass, decrease in cortical porosity, and increases in cortical/trabecular strength observed with DMAb treatment.
Disclosure:
J. D. Adachi,
Amgen Inc., Eli Lilly, Merck, Novartis,
5,
Amgen Inc., Eli Lilly, Merck, Novartis, Warner Chilcott,
7,
Amgen Inc., Eli Lilly, Merck, Novartis,
2;
S. Ferrari,
Amgen Inc., GSK, Lilly, MSD, Bioiberica,
5,
Novartis Pharmaceutical Corporation,
9,
Amgen Inc., MSD,
2;
C. Zapalowski,
Amgen Inc. ,
1,
Amgen Inc. ,
3;
P. D. Miller,
Amgen Inc., Lilly, Merck,
5,
Novartis Pharmaceutical Corporation,
9,
Warner-Chilcott,
7,
Amgen Inc., Lilly, Merck, Radius ,
2;
J. Y. Reginster,
Servier, Novartis, Negma, Lilly, Wyeth, Amgen Inc., GSK, Roche, Merckle, Nycomed, NPS, Theramex, UCB,
5,
Merck Sharp and Dohme, Lilly, Rottapharm, IBSA, Genevrier, Novartis, Servier, Roche, GlaxoSmithKline, Tejin, Teva, Ebewee Pharma, Zodiac, Analis, Theramex, Nycomed, Novo-Nordisk, Nolver,
8,
Bristol-Myers Squibb, Merck Sharp and Dohme, Rottapharm, Teva, Lilly, Novartis, Roche, GlaxoSmithKline, Amgen, Servier,
2;
O. Törring,
Amgen Inc., Takeda, GSK, Eli Lilly,
5;
N. Daizadeh,
Amgen Inc. ,
1,
Amgen Inc. ,
3;
A. Wang,
Amgen Inc. ,
1,
Amgen Inc. ,
3;
C. O’Malley,
Amgen Inc. ,
1,
Amgen Inc. ,
3;
R. B. Wagman,
Amgen Inc. ,
1,
Amgen Inc. ,
3;
E. M. Lewiecki,
Amgen Inc., Lilly, Merck ,
5,
Amgen, Lilly, Novartis ,
7,
Amgen, Lilly, Novartis ,
2.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/further-reduction-in-nonvertebral-fracture-rate-is-observed-following-3-years-of-denosumab-treatment-results-with-up-to-7-years-in-the-freedom-extension/