Session Information
Date: Sunday, October 26, 2025
Title: (0554–0592) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Axial spondyloarthritis (axSpA) is a chronic disease characterized by inflammatory low back pain and is often accompanied by inflammation of the peripheral joints and entheses. Patients receiving treatment with an IL-17 inhibitor, such as secukinumab (SEC), reported higher incidence of Candida and other fungal infections. The objective of this post hoc analysis is to describe the incidence, severity, seriousness, and type of fungal infections, including Candida, among patients with active axSpA treated with SEC.
Methods: This post hoc analysis included patients with active axSpA, including radiographic and non-radiographic disease, from a pooled population of 10 phase 3 clinical trials who received SEC 150 mg or placebo (PBO). Following the PBO-controlled period, patients randomized to PBO were switched to SEC 150 mg, while patients randomized to SEC 150 mg at baseline continued the same dose or were uptitrated to SEC 300 mg through the entire treatment period. Baseline demographic and clinical characteristics are reported in patients with or without ≥1 fungal infection adverse event during the entire treatment period. Incidence of fungal infections, including Candida, are reported for the PBO-controlled period and through the entire treatment period. Characteristics of infections including the specific types (defined by MedDRA preferred term), severity grades, resolution, and infections resulting in treatment discontinuation are reported through the entire treatment period.
Results: Overall, 2040 and 540 patients with active axSpA receiving SEC or PBO, respectively, were included. A total of 124 (6.1%) patients receiving SEC reported ≥1 fungal infection through the entire treatment period, and most (55.6%) were male. Male patients comprised 66.7% of the entire study population and the mean age was 41.3 years. During the PBO-controlled period, the incidence of total fungal infections were infrequent in each treatment group (exposure adjusted incidence rate [EAIR] per 100 patient-years [95% CI], SEC 150 mg: 5.51 [3.26-8.70], PBO: 3.39 [1.24-7.38], Table 1). EAIRs of Candida infections were also infrequent in patients receiving either SEC 150 mg: 3.05 (1.46-5.60) or PBO: 0.56 (0.01-3.13). Through the entire treatment period, the rates of total fungal infections (SEC 150 mg: 3.70 [3.01-4.50]; SEC 300 mg: 2.9 [1.73-4.76]) and Candida infections (SEC 150 mg: 1.30 [0.91-1.79]); SEC 300 mg: 0.68 [0.19-1.74]) did not increase. All fungal infections, including Candida, in patients receiving any dose of SEC through the entire treatment period were mild or moderate; no serious infections were reported (Figure 1). The vast majority (86.7% of total fungal infections and 87.1% of Candida infections) were fully resolved before the end of the treatment period (Figure 2), and 5 (3.0%) of infections resulted in treatment discontinuation.
Conclusion: Fungal infections, including Candida, were infrequent with incidence rates not increasing throughout the entire treatment period. Fungal infections during treatment with SEC were mild or moderate and most infections were fully resolved before the end of treatment, allowing almost all patients to continue treatment.
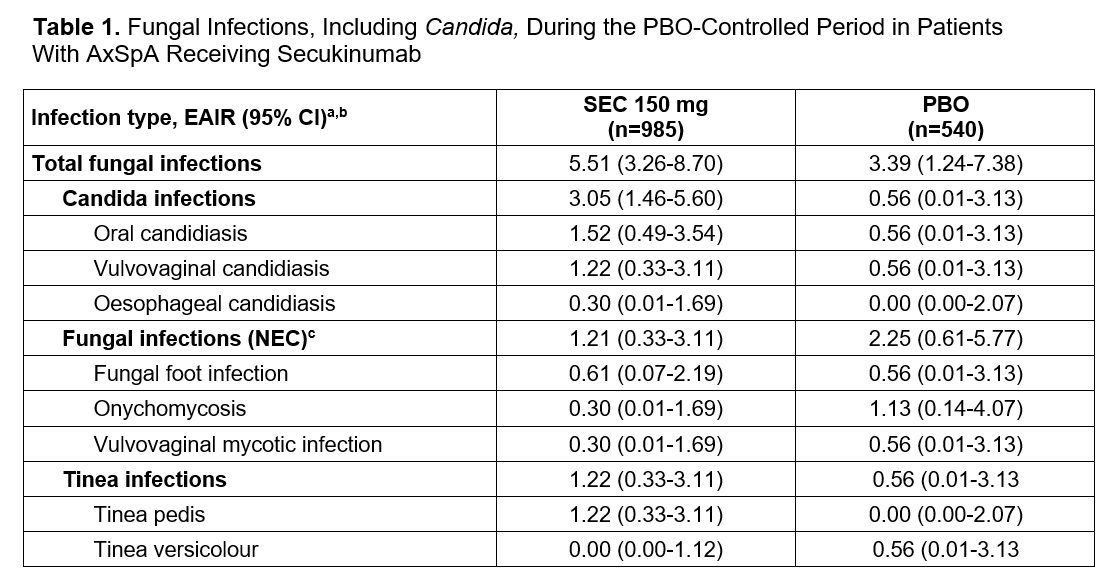 EAIR, exposure adjusted incidence rate per 100 patient-years; MedDRA, Medical Dictionary of Regulatory Activities; NEC, not elsewhere classifiable; PBO, placebo; SEC, secukinumab.
EAIR, exposure adjusted incidence rate per 100 patient-years; MedDRA, Medical Dictionary of Regulatory Activities; NEC, not elsewhere classifiable; PBO, placebo; SEC, secukinumab.
a MedDRA preferred term.
b A patient with multiple occurrences of an infection while receiving 1 treatment was counted only once in the infection category for that treatment.
c NEC denotes infections that were not specifically classified into any of the MedDRA preferred terms.
.jpg) PBO, placebo; SEC, secukinumab.
PBO, placebo; SEC, secukinumab.
a Infections are reported as the number of infections per treatment arm.
.jpg) PBO, placebo; SEC, secukinumab.
PBO, placebo; SEC, secukinumab.
a Total number of infections through the indicated time.
To cite this abstract in AMA style:
Singla S, Grinnell-Merrick L, Bao W, Zharkov A, Danve A. Fungal Infections, Including Candida, in Patients With Active Axial Spondyloarthritis Treated With Secukinumab: A Pooled Analysis of 10 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/fungal-infections-including-candida-in-patients-with-active-axial-spondyloarthritis-treated-with-secukinumab-a-pooled-analysis-of-10-phase-3-trials/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fungal-infections-including-candida-in-patients-with-active-axial-spondyloarthritis-treated-with-secukinumab-a-pooled-analysis-of-10-phase-3-trials/
