Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Objectives of long-term SLE management are not only to reduce disease activity, but also to prevent flares and minimize exposure to oral corticosteroids (OCS), which are associated with organ damage accrual.1,2 As tapering OCS increases flare risk, a therapy that both prevents flares and permits safe reductions of OCS is desirable. Flare rates were lower in patients with SLE who received anifrolumab vs placebo, and greater numbers of patients were able to taper OCS with anifrolumab vs placebo in the phase 3 trials, TULIP-1 and TULIP-2.3,4 We evaluated pooled TULIP trial data to further assess the capacity of anifrolumab to simultaneously prevent flares and permit OCS dosage reduction.
Methods: TULIP-1 and TULIP-2 were 52-week trials that evaluated the efficacy and safety of anifrolumab (300 mg IV Q4W for 48 weeks) or placebo in patients with moderately to severely active SLE despite standard-of-care treatment. Flares were defined as ≥1 new BILAG-2004 A or ≥2 new BILAG-2004 B domain scores vs the prior visit. Patients receiving OCS ≥10 mg/day at baseline were required to attempt tapering to ≤7.5 mg/day between Weeks 8 and 40; this reduction had to be sustained through Week 52. TULIP-1 and TULIP-2 were analyzed using restricted medication rules per the TULIP-2 protocol, and data from both trials were pooled. We analyzed flare occurrence in all patients, area under the curve (AUC) of OCS dosage among patients on OCS ≥10 mg/day at baseline, and flare rates among patients on OCS ≥10 mg/day at baseline who were able to sustain OCS dosage reduction to ≤7.5 mg/day from Week 40 to Week 52.
Results: Overall, 360 patients received anifrolumab and 366 received placebo. In total, fewer patients had ≥1 flare with anifrolumab vs placebo (33.6% vs 42.9%) (Table 1). In pooled TULIP data, 375/726 (51.7%) patients were taking OCS ≥10 mg/day at baseline; among these patients, OCS cumulative dosage through Week 52 was reduced with anifrolumab vs placebo (mean [SD] AUC 3947.1 mg [3655.5] vs 4275.8 mg [1859.0]). Among patients who were able to sustain OCS taper to ≤7.5 mg/day, fewer experienced ≥1 flare through Week 52 with anifrolumab vs placebo in pooled data (20.8% vs 45.8%) (Table 2). In contrast, in patients who were not able to taper OCS, there was no difference in the flare rates between anifrolumab and placebo (50.0% vs 51.6%). Overall, with anifrolumab, 76/190 (40.0%) patients were able to taper OCS without experiencing a flare through 52 weeks, compared with only 32/185 (17.3%) with placebo in pooled data.
Conclusion: In the phase 3 TULIP-1 and TULIP-2 trials, compared with placebo, anifrolumab was associated with reduced OCS cumulative dosage and, among patients who were able to taper and sustain OCS dosage to ≤7.5 mg/day to Week 52, a >2-fold reduction in flares. TULIP data support the capacity of anifrolumab to reduce flares while permitting the tapering of OCS, an important management goal for patients with SLE.
References
- Bonakdar ZS. J Res Med Sci. 2011;16:S427–32.
- Apostolopoulos D. Lancet Rheumatol. 2020;2:e24–30.
- Furie RA. Lancet Rheumatol. 2019;1:e208–19.
- Morand EF. N Engl J Med. 2020;382:211–21.
Writing assistance by Rosie Butler, PhD (JK Associates Inc., a Fishawack Health Company).
This study was sponsored by AstraZeneca.
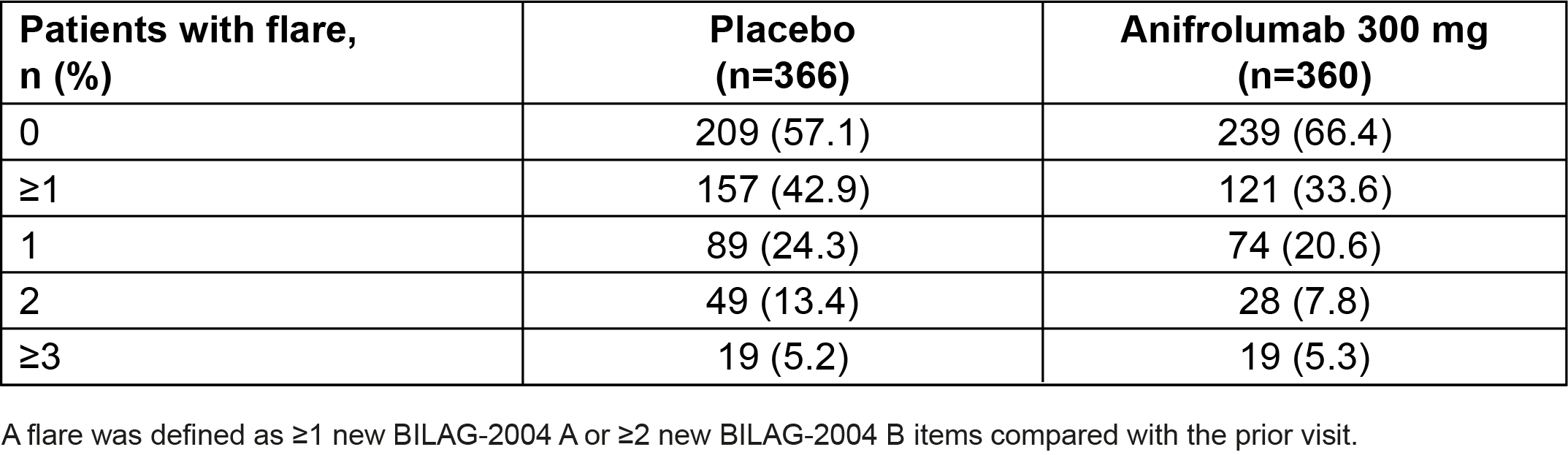 Table 1. Number of Flares Through Week 52 in Pooled Data From the TULIP-1 and TULIP-2 Trials
Table 1. Number of Flares Through Week 52 in Pooled Data From the TULIP-1 and TULIP-2 Trials
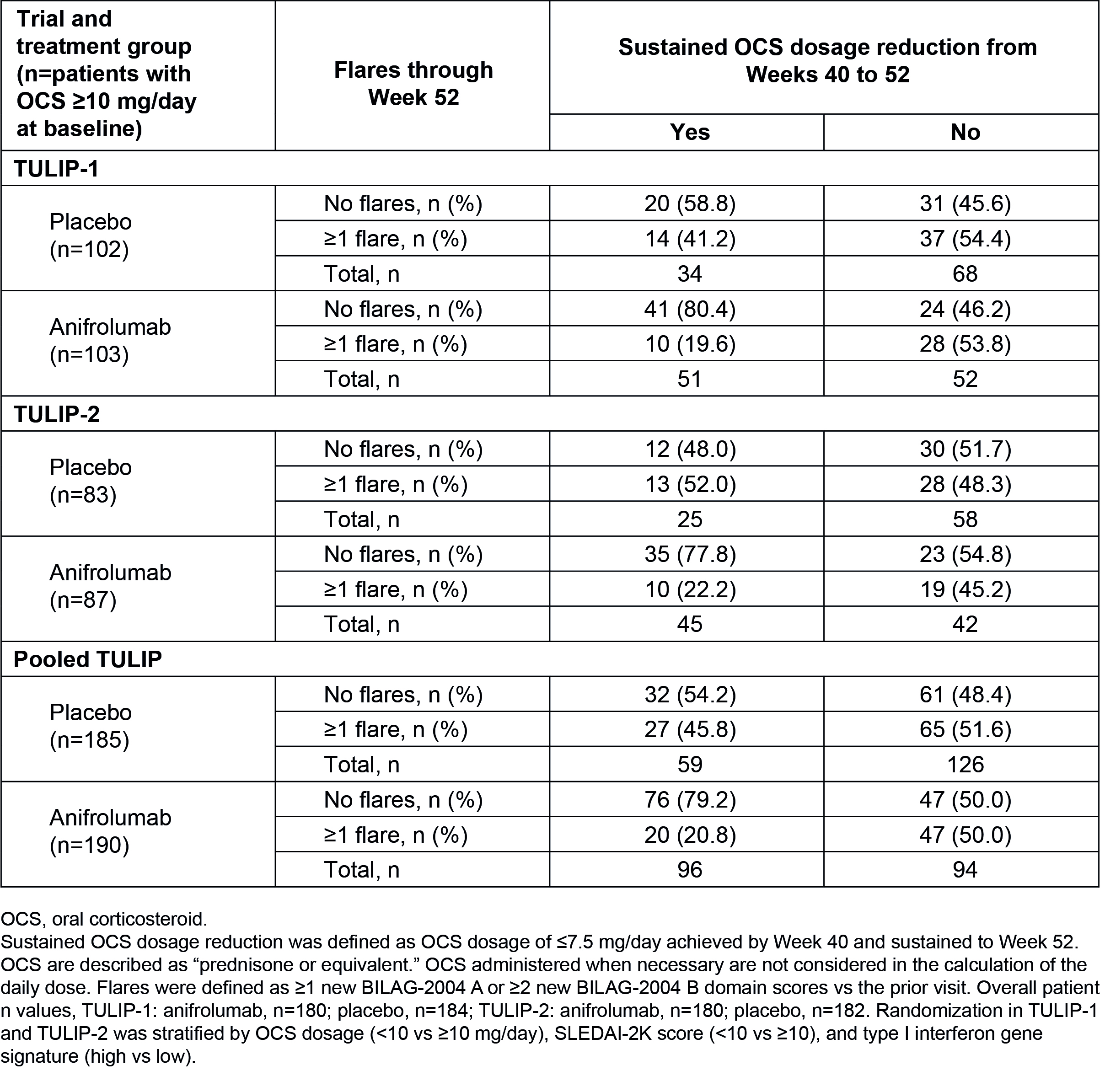 Table 2. Flares and Sustained OCS Dosage Reduction at Week 52 in Patients With OCS ≥10 mg/day at Baseline in TULIP-1, TULIP-2, and Pooled Data
Table 2. Flares and Sustained OCS Dosage Reduction at Week 52 in Patients With OCS ≥10 mg/day at Baseline in TULIP-1, TULIP-2, and Pooled Data
To cite this abstract in AMA style:
Furie R, Morand E, Askanase A, Vital E, Merrill J, Kalyani R, Abreu G, Pineda L, Tummala R. Flare Reduction and Oral Corticosteroid Taper in Patients with Active SLE Treated with Anifrolumab in 2 Phase 3 Trials [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/flare-reduction-and-oral-corticosteroid-taper-in-patients-with-active-sle-treated-with-anifrolumab-in-2-phase-3-trials/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/flare-reduction-and-oral-corticosteroid-taper-in-patients-with-active-sle-treated-with-anifrolumab-in-2-phase-3-trials/
