Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: Individuals with systemic lupus erythematosus (SLE) have an increased risk of infection compared to the general population. We aimed to assess the early risk of infection following rituximab therapy in a large national biologics register.
Methods: Patients receiving rituximab (RTX) or standard of care (SoC) treatment recruited to the UK national SLE biologics registry (2010-21) were included. Demographic, clinical and laboratory data were recorded at recruitment and during follow up. Information relating to infection and mortality were collected from study centres and the UK Office for National Statistics in the first 12-months after treatment initiation. Serious infections were defined as those requiring intravenous antibiotic treatment, hospital admission, resulting in disability or death.
Statistical analysis was performed using Stata (v14). Baseline variables were compared using Chi-squared or Mann-Whitney U tests. A logistic regression model adjusted for age and sex was used to test the association of baseline variables with serious infection within the first year.
Results: We included 931 patients (764 RTX and 167 SoC). In the SoC arm, commonest prescribed recent therapies included mycophenolate (n=126, 75%), azathioprine (n=46, 28%) and/or cyclophosphamide (n=38, 23%).
Baseline characteristics were similar between the two groups but RTX patients had a longer disease duration, higher number of previous immunosuppressants, more obstructive airways disease and lower maintenance steroid dose and baseline serum IgG (Table 1). In the first year following treatment initiation, 259 infections were reported in 143 (15%) patients, including 106 (41%) serious infections. In the RTX group, 127 (17%) individuals reported an infection compared to 16 (10%) in the SoC group, p=0.022. There was no difference in the number of serious infections between groups, 58 (8%) and 10 (6%) patients in RTX and SoC groups respectively, p=0.471. Recurrent serious infections occurred in 21 (2%) patients and were not significantly different between RTX and SoC groups (2.5% Vs 1.2%, p=0.309).
After adjusting for age and gender in the RTX group, number of comorbidities (OR 1.34 95% CI 1.08-1.65), maintenance steroid dose (OR 1.05 CI 1.01-1.08) and hypogammaglobulinaemia (IgG < 6.0 g/L) (OR 2.35, 95%CI 1.06-5.18) were associated with increased risk of infection (Table 2).
The commonest sites of serious infection were respiratory (n=24, 23%), bone/soft tissue (n=14, 13%), ENT (n=11, 10%) and urinary tract (n=8, 8%). One RTX-treated individual died of an infection (sepsis) within a year of treatment initiation.
Conclusion: RTX-treated SLE patients do not demonstrate an increased risk of serious infections compared to those on SoC treatment. Baseline hypogammaglobulinaemia, higher number of comorbidities and usual oral steroid dose correlate with infection risk in RTX-treated patients.
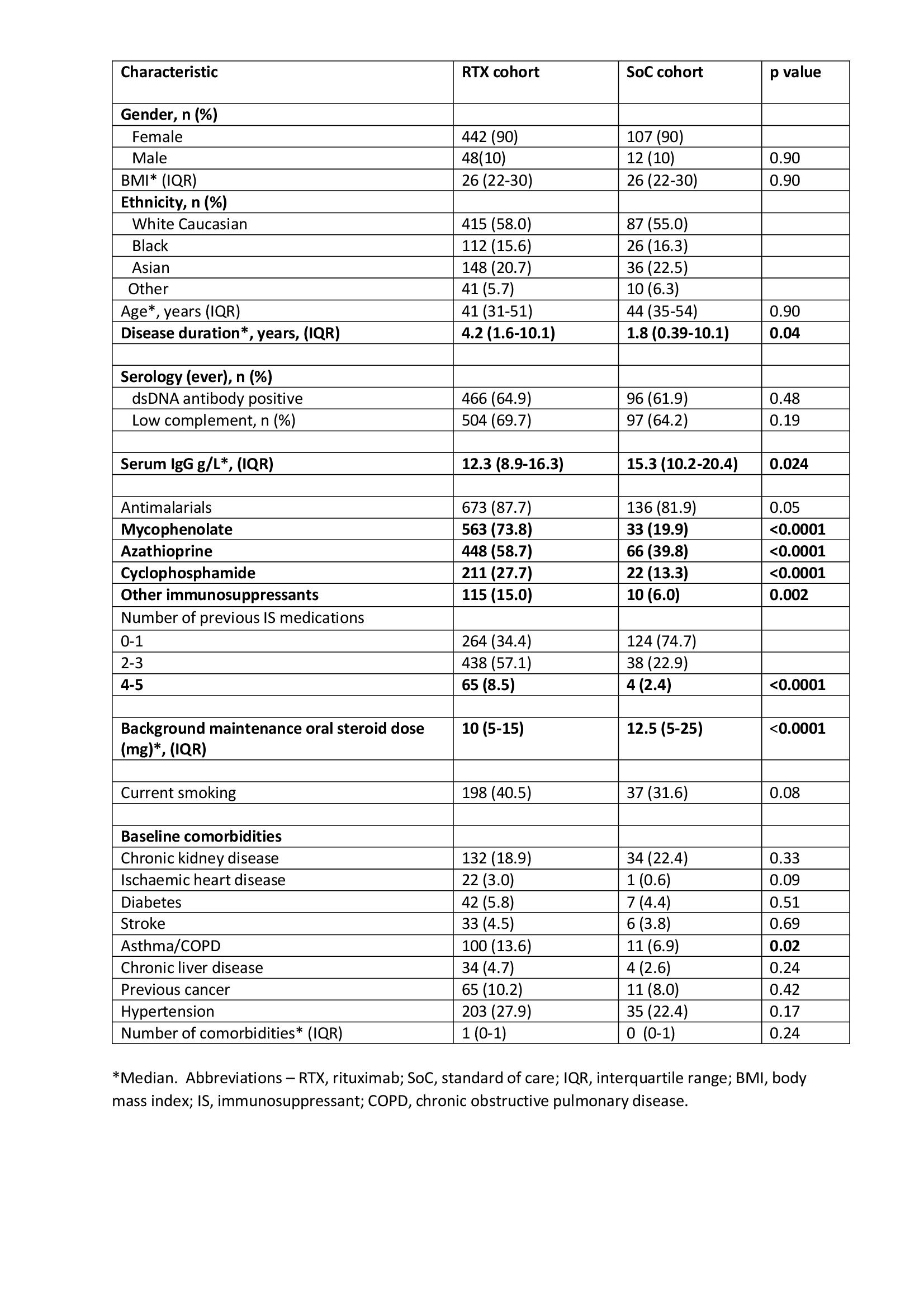 Table 1. Baseline characteristics of patients receiving rituximab and standard of care treatment
Table 1. Baseline characteristics of patients receiving rituximab and standard of care treatment
 Table 2. Clinical characteristics and their association with infection risk in rituximab treated patients
Table 2. Clinical characteristics and their association with infection risk in rituximab treated patients
To cite this abstract in AMA style:
Rodziewicz M, Dyball S, McDonald S, Sutton E, Parker B, Lupus Assessment Group T, Bruce I. First Year Infection Risk in SLE Patients Treated with Rituximab versus Standard of Care Treatment: Results from the British Isles Lupus Assessment Group Biologics Registry (BILAG-BR) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/first-year-infection-risk-in-sle-patients-treated-with-rituximab-versus-standard-of-care-treatment-results-from-the-british-isles-lupus-assessment-group-biologics-registry-bilag-br/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/first-year-infection-risk-in-sle-patients-treated-with-rituximab-versus-standard-of-care-treatment-results-from-the-british-isles-lupus-assessment-group-biologics-registry-bilag-br/
