Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: APS ACTION “Registry” was created to study the natural course of disease over 10 years in persistently antiphospholipid antibody (aPL)-positive patients with/without other systemic autoimmune diseases (SAIDx). The objective of this study was to update the incident first and recurrent thrombosis risk in persistently aPL-positive registry patients, previously reported in 2018 as 1.53% and 2.75%, respectively (Arthritis Rheumatol.2018;70[suppl 10]).
Methods: A web-based data capturing system is used to store patient demographics, history, and medications. The inclusion criteria are positive aPL according to Updated Sapporo Classification Criteria tested within one year prior to the enrollment. Patients are followed every 12±3m with clinical data and blood collection; they also receive counseling on cardiovascular disease (CVD) and venous thromboembolism (VTE) prevention at each visit. In this prospective analysis, based on patients who completed 1-8-year follow-up visits, we report the incident thrombosis risk in persistently aPL-positive patients without and with a history of thrombosis. We also compare the demographic and clinical characteristics of patients with and without new thrombosis.
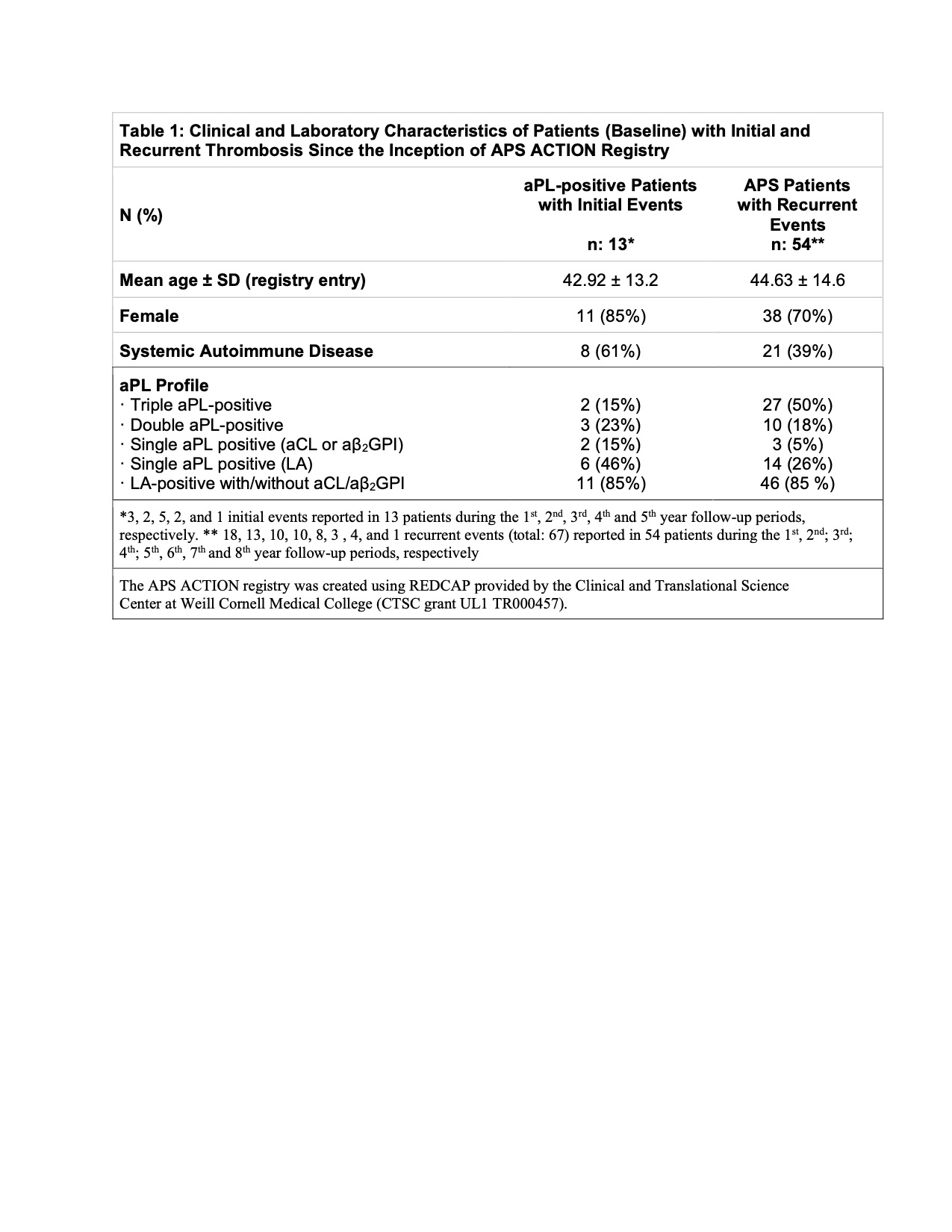
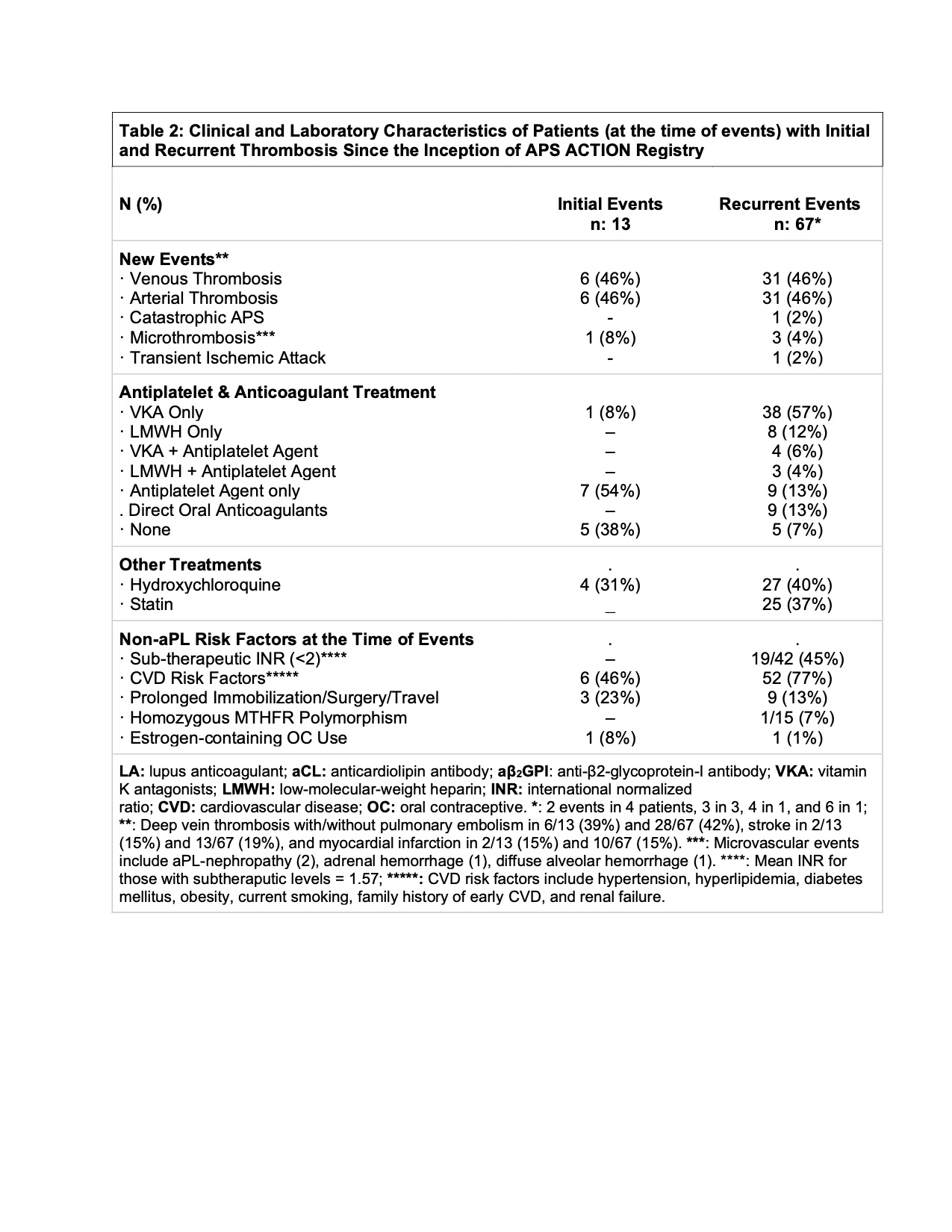
Results: As of March 2021, 886 patients were included: aPL/APS without SAIDx: 546 (aPL without APS classification: 101; thrombotic APS [TAPS]: 320; obstetric APS [OAPS]: 59; and TAPS+OAPS: 66]; and aPL/APS associated with SAIDx: 340 [aPL without APS: 91; TAPS: 178; OAPS: 21; and TAPS+OAPS: 50]). Of 886 patients, 705, 605, 527, 456, 401, 328, 200 and 49 completed 1, 2, 3, 4, 5, 6, 7, and 8-year follow-up, respectively. Mean follow-up was 4.67 years (1270 patient-years [pt-y]) and 4.19 years (2572 pt-y) for those without and with a history of thrombosis, respectively. Based on 13 initial events in 13 patients, and 67 recurrent events in 54 patients (Tables 1 and 2), the incident thrombosis risk was 1.02 and 2.09 per 100 pt-y in patients without and with a history of thrombosis, respectively. Demographics, concomitant lupus, aPL-profile, medications, and non-aPL thrombosis risk factors were not different between aPL-positive patients with (n=13) or without (n=259) first thrombosis, and between APS patients with (n=54) or without (n=560) recurrent thrombosis except APS patients with recurrence were younger and more likely to have triple aPL positivity compared to those without recurrence (42.1 ± 14.1 vs 46.6 ± 13.3, p 0.02, and 31% vs 50%, p 0.006).
Conclusion: Based on approximately 4000 patient-years of follow-up, the incident thrombosis risk in persistently aPL-positive patients remains relatively low (1.02 and 2.09 per 100 pt-y in patients without and with a history of thrombosis, respectively). Lupus anticoagulant and/or triple aPL-positivity, sub-therapeutic international normalized ratios, and additional CVD and VTE risk factors were relatively common at the time of the new events. Future Cox proportional hazards analysis will help us better define the risk and protective factors for thrombosis in persistently aPL-positive patients.
To cite this abstract in AMA style:
Ahmadzadeh Y, Andrade D, Tektonidou M, Pengo V, Sciascia S, Ugarte A, Belmont H, Gerosa M, Fortin P, Aguirre M, Ji L, Atsumi T, Cohen H, de Jesus G, Branch D, Tincani A, Kello N, Petri M, Rodriguez-Almaraz E, Ríos-Garcés R, Zuo Y, Artim-Esen B, Willis R, Bertolaccini M, Roubey R, Erkan D, APS ACTION o. First and Recurrent Thrombosis Risk After 3842 Patient-Years of Follow-Up: Prospective Results from AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository ( [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/first-and-recurrent-thrombosis-risk-after-3842-patient-years-of-follow-up-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-cli/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/first-and-recurrent-thrombosis-risk-after-3842-patient-years-of-follow-up-prospective-results-from-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-action-cli/