Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Up to 90% of patients with systemic sclerosis (SSc) have symptoms from the gastrointestinal (GI) tract. Earlier studies have shown a distinct alteration of the intestinal microbiome in SSc patients, compared to healthy adults. We therefore postulated that a normalization of the GI microbiota could improve some of the GI symptoms in this patient group. We aimed to determine the safety and efficacy of gut microbiota transplantation (GMT) in SSc patients and assess the effect on GI-symptoms and the general disease activity.
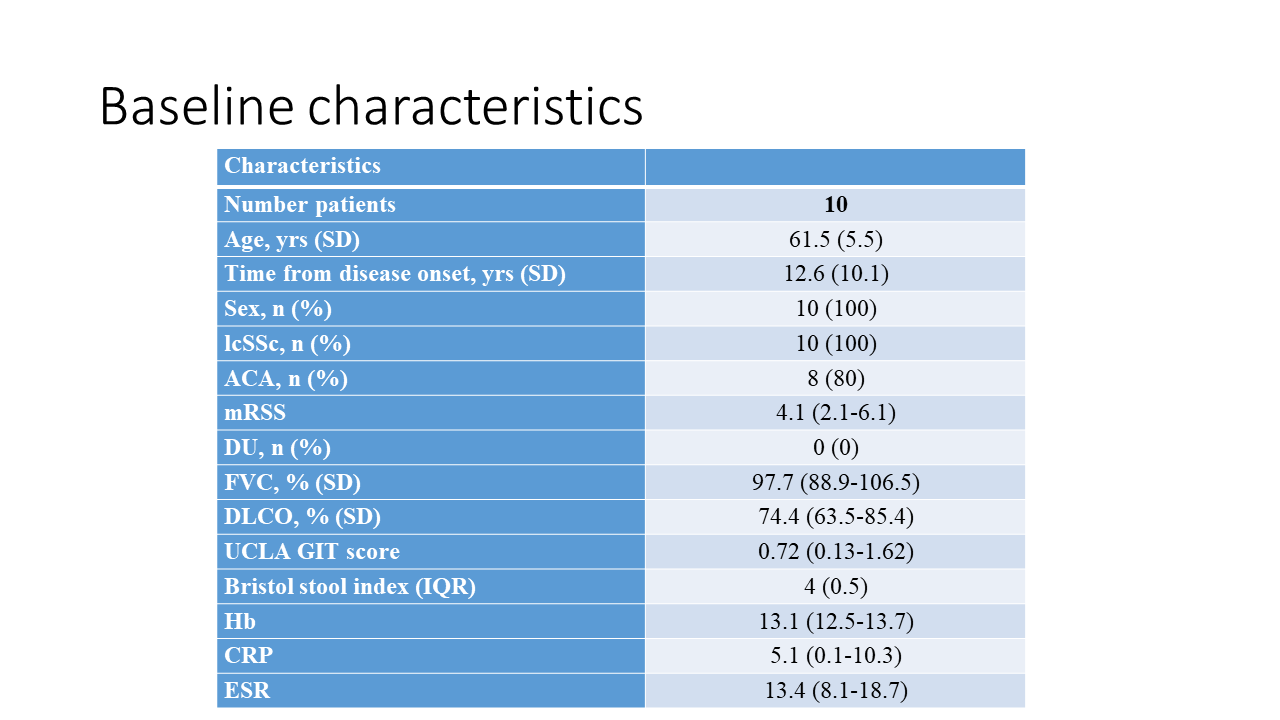
Methods: In this double-blind, placebo controlled pilot trial patients were randomized to the patented single-donor Anaerobically Cultivated Human Intestinal Microbiome (ACHIM) developed by ACHIM AB or placebo (culture media) (clinicaltrials.gov: NCT03444220). All participants had objective GI-involvement, were female >18 years old with limited cutaneous SSc and fulfilled the 2013 SSc classification criteria. Treatment was installed during upper gastroscopy twice with two weeks apart (week 0 and 2). Patients were followed for 16 weeks with six visits (week 0, 2, 4, 8, 12 and 16). GI-symptoms were reported using the ULCA-GIT score questionnaire. Primary end-point was defined as the minimally clinically important difference – the smallest change in score that patients perceive as beneficial. Disease activity was assessed by the validated SSc Disease Activity Index (DAI). Adverse events/safety were registered at each visit.
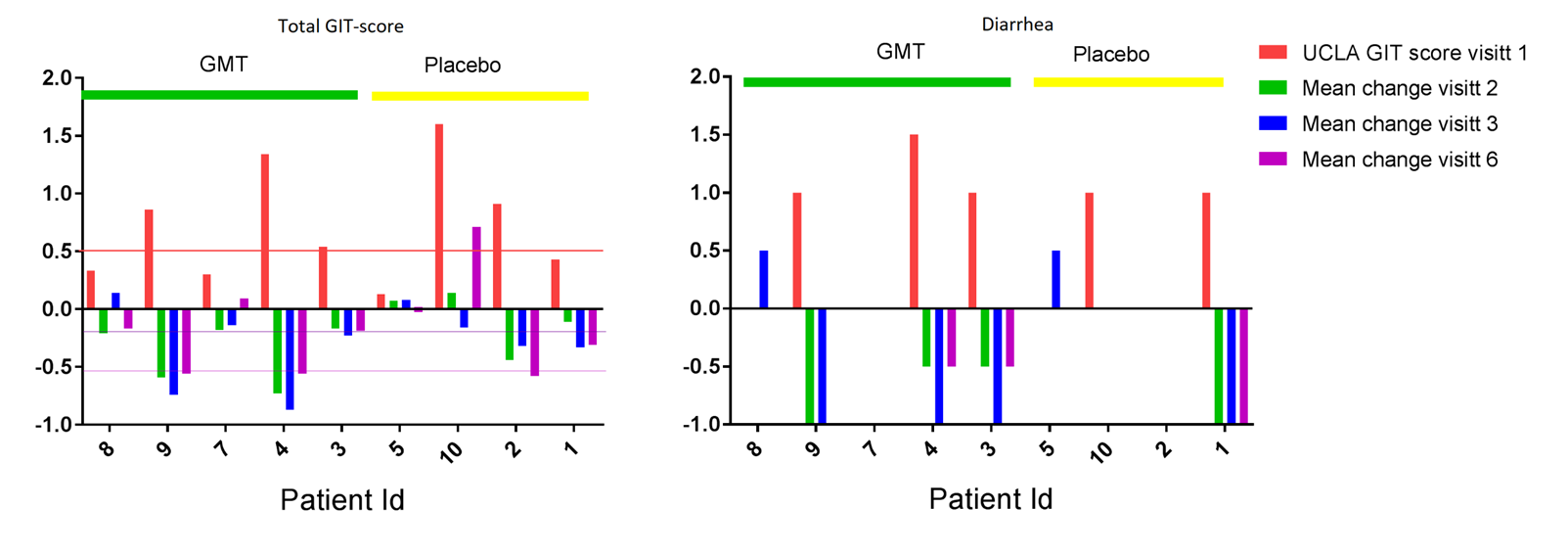
Results: Ten patients were enrolled and randomly assigned to GMT or placebo. One patient was excluded due to laryngospasms during the first gastroscopy. GMT was safe, mostly mild and short-term side effects were reported like abdominal bloating (n=4), intermittent diarrhea (n=4), nausea (n=1) and constipation (n=1). One patient suffered a severe side effect during biopsy with duodenal perforation, requiring intravenous antibiotics. Baseline and prospective changes in UCLA-GIT score are demonstrated in figure 1, showing clinical improvement in total UCLA GIT-score and diarrhea. Five patients reported fecal incontinence at visit 1; three received GMT and reported restoration of continence. DAI-score in the GMT group was 2.4 (SD 1.4) at week 0 and 2.1 (SD 1.9) at week 16. In the placebo group mean DAI at week 1 was 2.5 (SD 1.5) and 1.8 (SD 1.5) at week 16.
Conclusion: The first double-blind, randomized clinical trial on GMT in SSc patients shows promising effects on GI symptoms. There was a clinically meaningful improvement in diarrhea and fecal incontinence after treatment with GMT. Larger trials are needed to confirm the results of this pilot study.
To cite this abstract in AMA style:
Fretheim H, Midtvedt O, Heiervang Tennøe A, Didriksen H, Garen T, Bækkevold E, Hov JR, Lundin KE, Trøseid M, Molberg Ø, Hoffmann-Vold AM. Fecal Microbiota Transplantation in Patients with Systemic Sclerosis- a Pilot Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/fecal-microbiota-transplantation-in-patients-with-systemic-sclerosis-a-pilot-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/fecal-microbiota-transplantation-in-patients-with-systemic-sclerosis-a-pilot-study/