Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: SWITCH-RA is a global, observational study evaluating the effectiveness of switching to an alternative TNFi or rituximab (RTX) following initial TNFi failure in patients with RA. The study also assessed the reasons for discontinuation of initial TNFi therapy and factors that drive choice of RTX vs alternative TNFi as subsequent therapy.
Methods: Reasons for initial TNFi discontinuation and rationale for selection of subsequent therapy were recorded. The association between various factors and choice of RTX or alternative TNFi as second biologic were analyzed using logistic regression with a stepwise method for variable selection.
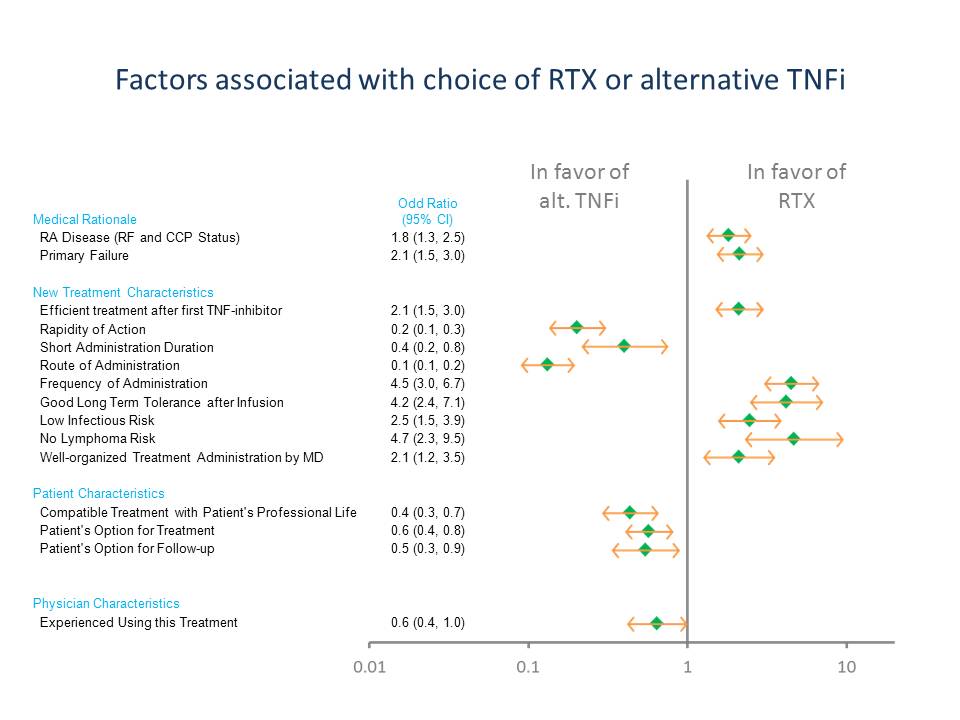
Results: A total of 1107 enrolled patients (mean age 55.5 yrs; mean disease duration 8.3 yrs) were analyzed. Reasons for discontinuing initial TNFi were: lack of efficacy (n=824 [74%]); intolerance (n=264 [24%]); other (n=19 [2%]). In all, 602 (54%) received RTX and 505 (46%) an alternative TNFi as second biologic. Factors associated with choice of RTX or alternative TNFi are shown in the Figure.
Factors most clearly associated with selection of RTX were generally associated with treatment characteristics related to the safety profile (no lymphoma risk, low infection risk, and good long-term tolerance after infusion) and frequency of administration. Factors most clearly associated with selection of an alternative TNFi were associated with treatment (route of administration, rapidity of action, short administration duration) and patient (treatment being compatible with patient’s professional life) characteristics.
Conclusion: Lack of efficacy was the main reason for discontinuation of an initial TNFi. Factors associated with selection of RTX over an alternative TNFi tended to be associated with treatment characteristics related to the safety profile and frequency of administration, while those associated with selection of an alternative TNFi were associated with administration and patient characteristics.
Disclosure:
A. Finckh,
Roche, Pfizer, BMS,
2,
Roche, BMS, Pfizer,
5;
J. E. Gottenberg,
Roche, Pfizer, MSD, Abbott,
5;
C. Mpofu,
F Hoffmann-La Roche Ltd,
3;
W. G. Bensen,
Abbott, Amgen, AstraZeneca, BMS, Merck-Schering, Janssen, Lilly, Novartis, Pfizer and Wyeth, Proctor and Gamble, Roche, Sanofi-Aventis, Servier, UCB, Warner Chilcott,
2,
Abbott, Amgen, AstraZeneca, BMS, Merck-Schering, Janssen, Lilly, Novartis, Pfizer and Wyeth, Proctor and Gamble, Roche, Sanofi-Aventis, Servier, UCB, Warner Chilcott,
5,
Abbott, Amgen, AstraZeneca, BMS, Merck-Schering, Janssen, Lilly, Novartis, Pfizer and Wyeth, Proctor and Gamble, Roche, Sanofi-Aventis, Servier, UCB, Warner Chilcott,
8;
A. Rubbert-Roth,
Roche, Pfizer,
2,
Roche, Chugai, Abbott, Pfizer, UCB, MSD,
5,
Roche, UCB, Chugai. MSD,
6,
Roche, UCB, Chugai, MSD,
8;
F. Irazoque,
Pfizer, Roche, Janssen,
5;
V. Martínez Taboada,
Schering-Plough, Wyeth-Pharma, Roche,
2,
UCB-Pharma, Bristol Myers Squibb, Roche, Cellerix, Pfizer,
5;
C. Chung,
Genentech, Inc (full time),
3;
L. Hinsch-Gylvin,
F Hoffmann-La Roche Ltd,
3;
C. Ferri,
None;
P. Emery,
Pfizer, Merck, Abbott, BMS, Roche, UCB,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-influencing-choice-of-rituximab-versus-an-alternative-tumor-necrosis-factor-inhibitor-following-tumor-necrosis-factor-inhibitor-failure-in-patients-with-rheumatoid-arthritis-sub-analysis-of-a/