Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Tocilizumab (TCZ) is the only biological drug approved for the treatment of giant cell arteritis (GCA). Clinical trials were performed with intravenous (IV) and subcutaneous (SC) TCZ in a phase-2, and phase-3 (GiACTA) studies, respectively. Interestingly, around a quarter of GCA patients have a relapse during TCZ treatment. Our aim was to assess the factors associated with relapses in a wide series of GCA patients in a real-world setting.
Methods: Multicenter observational study of 407 patients with GCA treated with TCZ. The diagnosis of GCA was performed between 2016 and 2021 according to: a) 1990 ACR criteria, b) temporal artery biopsy, and/or c) imaging evaluation techniques. The variables collected at TCZ initiation were demographics, clinical manifestations, laboratory, temporal artery biopsy, imaging findings, prednisone-equivalent dose and previous therapies. Patients with and without relapses were compared in a bivariate analysis. Multivariate logistic regression was performed to determine factors associated with relapse.
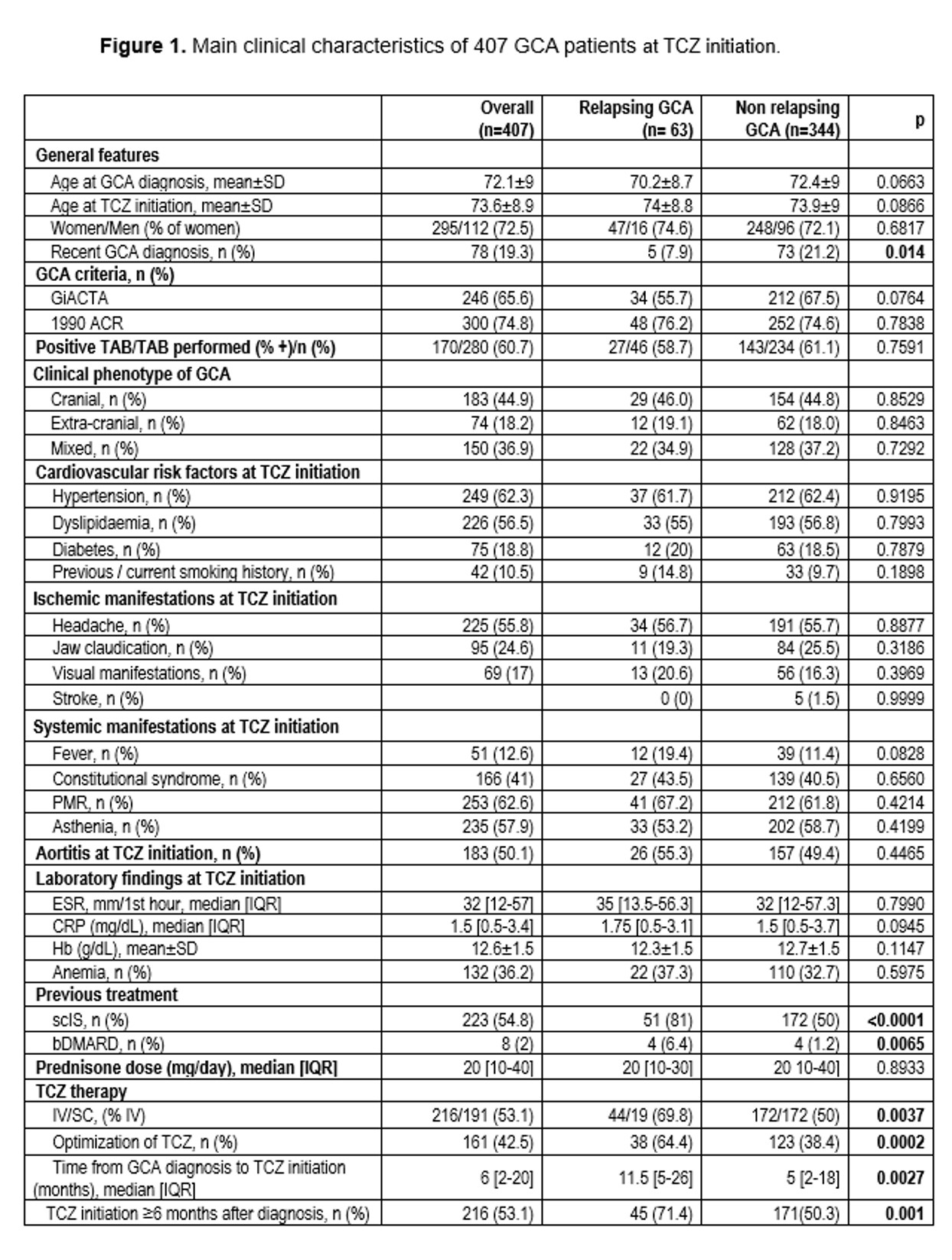
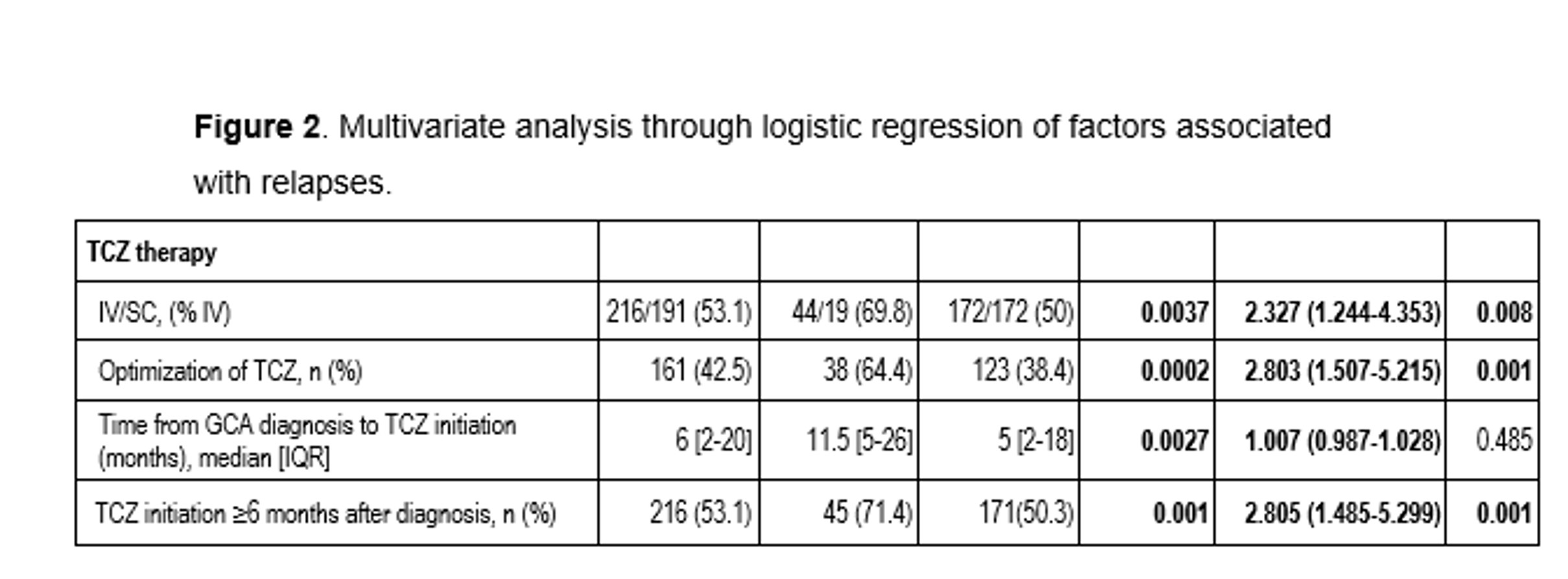
Results: After a mean follow-up of 25.3±21.7 months, GCA relapses were observed in 63 of 407 (15.5%) patients. No significant differences were observed between both groups (with/without relapses) in demographic, clinical and laboratory characteristics or in prednisone dose at initiation of TCZ. Interestingly, the set of variables associated with GCA relapses were: use of IV TCZ, combination of TCZ, optimization of TCZ and TCZ initiation ≥6 months after diagnosis. (Figure 1,2)
Conclusion: GCA relapse seems related mainly to TCZ schedule including IV use, combination use of TCZ, as well as, optimization of TCZ and initiation of TCZ after 6 months of diagnosis.
Results were adjusted by the variables: “age at TCZ initiation”, “fever at TCZ initiation”, “previous/current smoking history at TCZ initiation”, “Hb at TCZ initiation”. Statistical significance is specified as bold characters.
To cite this abstract in AMA style:
Martín-Gutiérrez A, Loricera J, Secada Gómez C, Moriano C, Narvaez-García J, Aldasoro V, Maíz Alonso O, Hernández-Rodríguez I, Vela-Casasempere P, Romero Yuste S, De Miguel E, Galindez-Agirregoikoa E, Fernandez-López J, Ferraz-Amaro i, Sánchez-Martín J, Callejas J, Moya Albarado P, Campos Fernández C, Lopez-Gutierrez F, Castañeda S, Blanco-Alonso R. Factors Associated with Relapse in Giant Cell Arteritis Treated with Tocilizumab. Multicenter Open-label Study of 407 Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/factors-associated-with-relapse-in-giant-cell-arteritis-treated-with-tocilizumab-multicenter-open-label-study-of-407-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/factors-associated-with-relapse-in-giant-cell-arteritis-treated-with-tocilizumab-multicenter-open-label-study-of-407-patients/