Session Information
Date: Saturday, November 6, 2021
Title: Miscellaneous Rheumatic & Inflammatory Diseases Poster I (0183–0209)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: There is a lack of robust guidance and consistently effective treatment modalities in non-paraneoplastic autoimmune retinopathy (npAIR) with cystoid macula edema (CME), which is often jointly treated by the Rheumatology provider. This retrospective case series was generated as further evidence is needed to understand options for treatment to include the effect of anti-interleukin-6 (IL-6) receptor monoclonal antibodies Tocilizumab (humanized monoclonal antibody) and Sarilumab (fully humanized monoclonal antibody) in eyes with npAIR with CME.
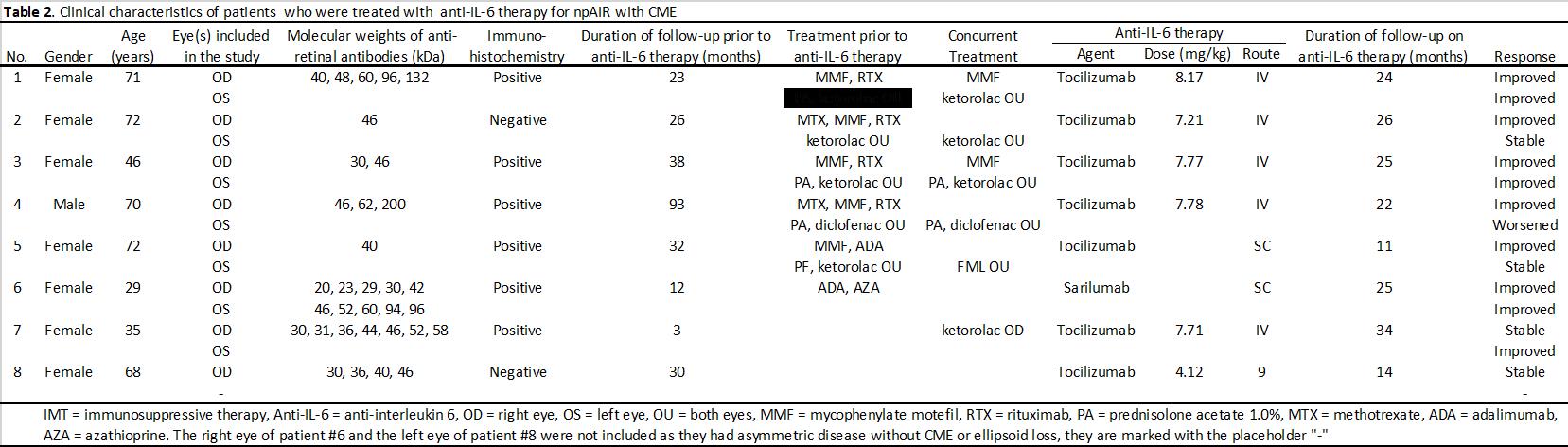
Methods: This retrospective case series included Fourteen eyes from 8 patients with npAIR and CME, all were seropositive for anti-retinal antibodies, treated with anti-IL-6 therapy and had a negative workup for paraneoplastic etiologies. Visual acuity (VA) and central subfield thickness (CST), total macular volume (TMV), and ellipsoid zone integrity change in central 6mm on foveal scan as measured by optical coherence tomography were extracted from charts prior to and at 3, 6, 12, 18, and 24 months after anti-IL-6 therapy was initiated. Eyes that had a >20% reduction in CST were defined as treatment responders, >20% increase in CST as failures, and ≤20% change in CST as stable. The main outcome measures were VA CST, TMV, and ellipsoid zone integrity change.
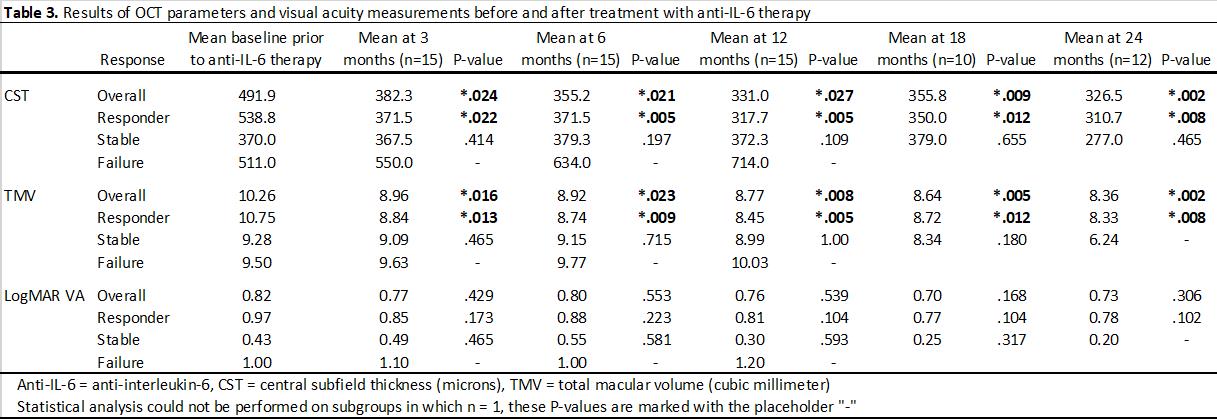
Results: Eleven eyes of six patients had failed multiple prior immunosuppressive therapies (Table 2). There was a significant reduction (P< .05) in CST at baseline (473.1 mm) after initiation of anti-IL-6 therapy that began at 3 months (389.2 mm) and continued through 24 months (348.7 mm). Similarly, there was a significant reduction (P< .05) in TMV baseline (10.15 mm3) after initiation of anti-IL-6 therapy that began at 3 months (8.91 mm3) and continued through 24 months (7.67 mm3). VA improved from 0.84 to 0.52 at 24-month follow-up but did not reach statistical significance (P=.157). Nine of 14 eyes (64.3%) were treatment responders, 4 eyes (28.6%) were stable, and 1 eye (7.1%) was a treatment failure. After initiation of IL-6 therapy, there was a significant decrease in velocity of EZ change, experiencing an average restoration of 22.7 mm per month (P=.008). At least one eye of all patients responded to anti-IL-6 treatment. No patient experienced adverse effects or required discontinuation of therapy while on anti-IL-6 medication.
Conclusion: In this cohort of patients with npAIR and CME, treatment with anti-IL-6 medications tocilizumab and sarilumab, was associated with reduced CME, partial restoration of the ellipsoid zone, and a trend towards improved VA, while reducing burden of prior immunosuppressive therapies (Table 3). The treatment was well tolerated and these largely positive and sustained results are important given the limited number of viable treatment options in the management of npAIR.
To cite this abstract in AMA style:
Carnago L, Keenan R, Jaffe G, Deaner J, Grewal D. Eye Opening: Use of IL-6 Inhibition in Non-Paraneoplastic Autoimmune Retinopathy [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/eye-opening-use-of-il-6-inhibition-in-non-paraneoplastic-autoimmune-retinopathy/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/eye-opening-use-of-il-6-inhibition-in-non-paraneoplastic-autoimmune-retinopathy/