Session Information
Date: Monday, October 22, 2018
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: AIDAI is a novel and unique, validated patient (pt)-reported assessment tool to evaluate disease activity in familial Mediterranean fever (FMF), hyper-IgD syndrome/mevalonate kinase deficiency (HIDS/MKD) and TNF receptor-associated periodic syndrome (TRAPS).1 Here we performed an external validation of AIDAI by calculating scores over 40 weeks (wks) of canakinumab (CAN) treatment in pts enrolled into the CLUSTER trial (NCT02059291) and assessed correlation between AIDAI and disease/response characteristics.
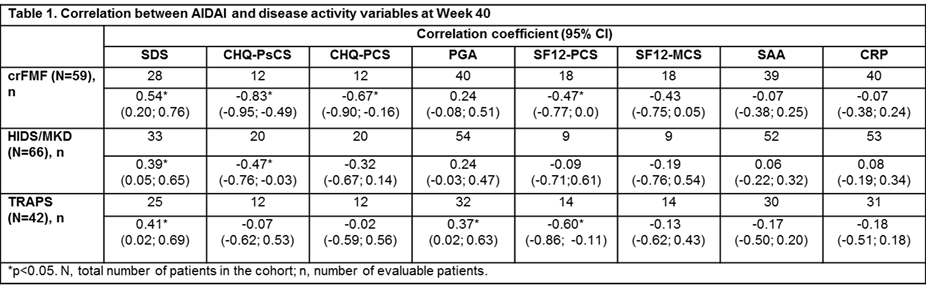
Methods: CLUSTER consisted of one cohort per disease (crFMF, HIDS/MKD and TRAPS).2 AIDAI was calculated as the sum of 12 items (Yes=1; No=0)1 for 30 consecutive days. AIDAI score was calculated if the first score was recorded before ≥29 days. Missing items beyond last evaluable measurement were imputed by last observation carried forward (LOCF). Inactive disease (ID) was defined as AIDAI score <9. Correlation analysis of AIDAI with Sheehan disability score (SDS), child health questionnaire–psychological/physical (CHQ–PsCS/PCS), physician global assessment (PGA), short form 12–physical/mental component summaries (SF12–PCS/MCS), C-reactive protein (CRP), and serum amyloid A (SAA) were performed. Significance was set at p<0.05.
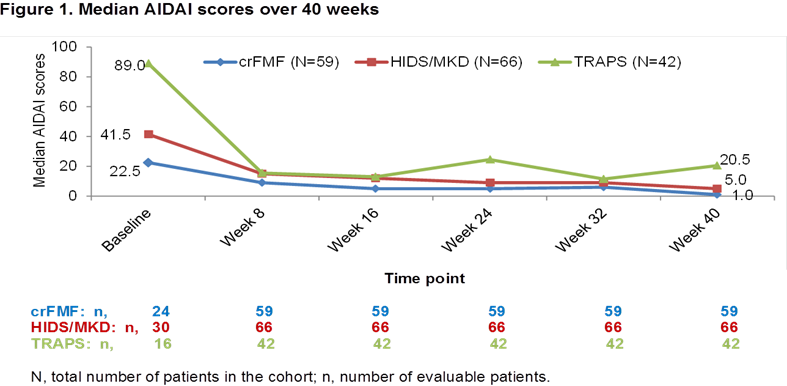
Results: Overall, 167 (crFMF: N=59; HIDS/MKD: N=66; TRAPS: N=42) pts were randomized to CAN 150 mg or placebo every 4 wks. Median AIDAI scores in all 3 cohorts decreased from baseline (BL) to Wk 16 (crFMF: 22.5 to 5.0; HIDS/MKD: 41.5 to 12.0; TRAPS: 89.0 to 13.0) and through Wk 40 (crFMF: 1.0; HIDS/MKD: 5.0; TRAPS: 20.5; Fig 1). In all 3 cohorts, the proportion of pts with ID (AIDAI score <9) was higher at Wk 40 versus BL (crFMF: 69.5% vs 5.1%; HIDS/MKD: 56.1% vs 6.1%; TRAPS: 42.9% vs 2.4%). AIDAI at Wk 40 correlated significantly with: SDS in all 3 cohorts; CHQ-PsCS in crFMF and HIDS/MKD; CHQ-PCS in crFMF; PGA in TRAPS; SF12–PCS in crFMF and TRAPS. SF12-MCS, CRP, and SAA did not correlate with AIDAI (Table 1).
Conclusion: AIDAI scores decreased markedly over 40 weeks of treatment with canakinumab in crFMF, HIDS/MKD and TRAPS, with a relevant percentage of patients having inactive disease score. AIDAI improvements at Week 40 correlated with patient- and physician-driven evaluations. AIDAI is a validated patient-reported tool to assess disease activity and appears to have good sensitivity to change to be used in comparative trials. Patient’s experience on disease activity does not strictly correlate with CRP and SAA, as these reflect more closely biological inflammation than clinical symptoms.
References: 1. Piram M, et al. Ann Rheum Dis. 2014;73:2168-73. 2. De Benedetti F, et al. NEJM. 2018;378:1908-19.
To cite this abstract in AMA style:
Koné-Paut I, Piram M, Benseler S, Kuemmerle-Deschner JB, Jansson AF, Rosner I, Tommasini A, Murias S, Karadag O, Levy J, Smeets S, De Benedetti F. External Validation of the Autoinflammatory Disease Activity Index (AIDAI) in Patients with Colchicine-Resistant FMF, Hids/Mkd, and TRAPS: Results from a Pivotal, Phase 3 Trial of Canakinumab [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/external-validation-of-the-autoinflammatory-disease-activity-index-aidai-in-patients-with-colchicine-resistant-fmf-hids-mkd-and-traps-results-from-a-pivotal-phase-3-trial-of-canakinumab/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/external-validation-of-the-autoinflammatory-disease-activity-index-aidai-in-patients-with-colchicine-resistant-fmf-hids-mkd-and-traps-results-from-a-pivotal-phase-3-trial-of-canakinumab/