Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The 2019 EULAR/ACR Classification Criteria allow classification of patients with systemic lupus erythematosus (SLE) for research. They reflect updates in current understanding of SLE and their value has been extensively documented in traditional research cohorts. Their functionality in electronic healthcare records is not well established. The purpose of this study was to describe the recording of the 2019 EULAR/ACR Classification Criteria within a UK electronic health database, the Clinical Practice Research Datalink (CPRD) to aid understanding of their utility in research studies in this setting.
Methods: Ethical approval was obtained from the CPRD Independent Scientific Advisory Committee (21_000697). The study period was 01/01/1990 to 31/12/2020. Read code lists were devised to identify adult patients with possible SLE within the CPRD, defined as cases. Codes relating to discoid, tuberculous or drug-induced lupus were excluded. Valid patients within the CPRD without a record of a SLE code were defined as non-cases. EULAR/ACR criteria definitions were adapted for use within the CPRD. Patient records were searched for the presence of criteria items. Descriptive statistics were used to describe the capture of ACR criteria for patients. Odds ratios were used to compare the presence of individual criteria in cases vs non-cases.
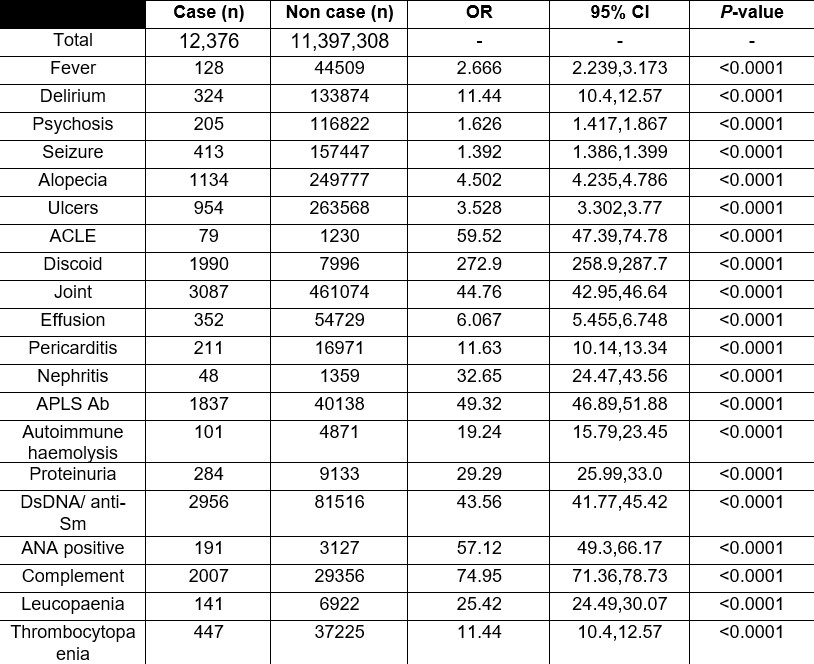
Results: 12,376 cases and 11,397,308 non-cases were identified. Counts for each criteria, for both groups, are presented in Table 1. Only 1.5% (n=191) of cases had a record of a positive ANA test. The most common criterion seen for cases was joint involvement (24%, n=3087, and the least common was autoimmune haemolysis (0.8%, n=101). Odds ratios and 95% confidence intervals for the presence of criteria items in cases and non-cases are presented in Table 1. The odds of all EULAR/ACR criteria items were significantly higher in cases than non-cases. This was most pronounced for discoid rash (OR 272.9, 258.9-287.7, P< 0.0001) and least for seizure (OR 1.392, 1.386-1.399,P< 0.0001).
Conclusion: These data show that the 2019 EULAR/ACR SLE criteria can be identified within the CPRD. The prevalence of these items is lower than those seen in traditional research studies which reflects differences in recording practices between primary and secondary care. Researchers should be cautious when using absolute counts of these items within the CPRD and possibly other primary care databases. Low prevalence of ANA positivity will preclude this as an entry criteria in this setting. The higher odds of criteria in cases compared to non-cases, suggests use of Read codes is likely to identify true SLE cases; combining these with 2019 EULAR/ACR SLE criteria and other parameters may further aid this process.
To cite this abstract in AMA style:
Ellis J, McGrogan A, McHugh N, Mulhearn B, Korendowych E, Pauling J, Bruce I, Humphreys J, Skeoch S. Extent of Recording of 2019 EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in a UK Healthcare Database [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/extent-of-recording-of-2019-eular-acr-classification-criteria-for-systemic-lupus-erythematosus-in-a-uk-healthcare-database/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/extent-of-recording-of-2019-eular-acr-classification-criteria-for-systemic-lupus-erythematosus-in-a-uk-healthcare-database/