Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Psoriatic arthritis (PsA) is a chronic systemic inflammatory musculoskeletal disease. The potential relationship between PsA therapies and specific safety events such as major adverse cardiovascular events (MACE), malignancy, thrombotic events, and serious opportunistic infections (SOIs) has the potential to limit long-term use. Apremilast (APR), an oral phosphodiesterase-4 inhibitor, modulates pro-inflammatory and anti-inflammatory mediators, differentiating it from biologics and the class of JAK inhibitors. We report the integrated pooled safety data of APR in patients with PsA from 5 clinical studies with a focus on long-term adverse events (AEs) of special interest.
Methods: Data from 5 randomized, placebo (PBO)-controlled, phase 3 studies (PALACE1–4 and ACTIVE) in patients with active PsA who met the Classification Criteria for Psoriatic Arthritis were pooled. The PBO-controlled phase lasted up to 24 weeks, after which all patients received APR for the remainder of the study with a total exposure up to 5 years. PBO data presented are for the PBO-controlled phase. APR data are for the PBO-controlled period and APR exposure period, which includes all patients’ data during their APR exposure period, regardless of when patients started APR treatment. Pooled analysis assessments included the exposure-adjusted incidence rate (EAIR) per 100 person years (PY) for treatment-emergent AEs (TEAEs) of special interest, including MACE, thrombotic events, malignancies, and SOIs.
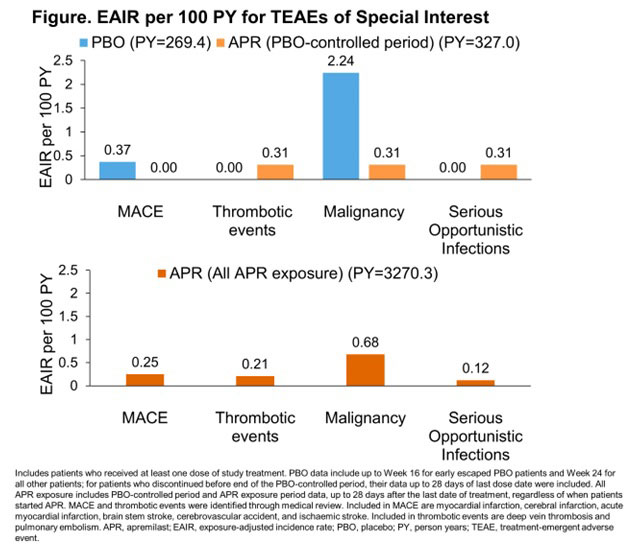
Results: During the PBO-controlled period, 780 patients received PBO, 781 received APR, and 1179 received APR during any period of study treatment. Overall APR exposure was 3270 PY; median exposure was 117 weeks. At baseline, mean age was 50 years, 53.4% were women, and 95.6% were white. Mean body mass index was 30 kg/m2 and mean duration of PsA was 6 years at baseline. Baseline demographics and disease characteristics were similar between APR- and PBO-treated patients. During the PBO-controlled period, EAIRs/100 PY of TEAEs were 212.9 (PBO) and 275.6 (APR) (Table). Most TEAEs were mild to moderate in severity (serious TEAEs EAIRs: PBO 11.3/100 PY; APR 7.1/100 PY) and discontinuation due to TEAEs was low (EAIRs: PBO 15.7/100 PY; APR 16.1/100 PY during the PBO-controlled period) (Table). No deaths were reported in the PBO-controlled period. EAIRs per 100 PY for MACE and malignancy were higher for PBO than for APR (Figure). Thrombotic events and SOI EAIRs were lower for PBO than APR based on a small number of cases (Figure). In the all APR exposure period, EAIR/100 PY of TEAEs was 114.8 and EAIRs/100 PY for serious TEAEs and discontinuations due to TEAEs were 6.6 and 4.1, respectively (Table). Five deaths, deemed unrelated to study drug, were reported in the all APR exposure period (0.2 EAIR/100 PY).
Conclusion: In patients with active PsA, long-term use of APR did not signal potential risk of MACE or malignancy. EAIRs for these events were lower with APR vs PBO for the PBO-controlled period. EAIR for thrombotic events and SOIs was low over the 5-year treatment period. APR has an established long-term safety profile and is an important therapeutic option for patients with PsA.
To cite this abstract in AMA style:
Mease P, Gladman D, Schett G, Paris M, Cheng S, Richter S, Teng L, Kavanaugh A. Exposure-Adjusted Incidence Rate for Adverse Events of Special Interest in Patients with Psoriatic Arthritis Treated with Apremilast [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/exposure-adjusted-incidence-rate-for-adverse-events-of-special-interest-in-patients-with-psoriatic-arthritis-treated-with-apremilast/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exposure-adjusted-incidence-rate-for-adverse-events-of-special-interest-in-patients-with-psoriatic-arthritis-treated-with-apremilast/