Session Information
Date: Monday, November 18, 2024
Title: Abstracts: SpA Including PsA – Diagnosis, Manifestations, & Outcomes II
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Previous studies have reported weight gain in Psoriatic arthritis (PsA) with biologics like TNF inhibitors (i). In contrast, no significant increase in body weight was found among patients receiving conventional synthetic (cs)DMARDs, Interleukin (IL)-12/23i, and IL-17i. These studies focused on absolute weight differences, with the drawbacks of smaller sample sizes and failure to adjust for other factors. We aimed to study the change in weight with the use of csDMARDs, biologic (b)DMARDs, Janus Kinase i (JAKi), and apremilast, along with other factors affecting weight across the cohort.
Methods: Patients receiving NSAIDs or no medications, csDMARDs only (with or without NSAIDs), TNFi as the first biologic, anti-IL12/23i, anti-IL17i, IL23i, JAKi, and apremilast at any point more than a year were identified from the database of a large cohort. The weight trend before and after each drug was studied by adjusting for age, sex, disease duration, comorbidities, disease activity (PASI and swollen joint count-SJC), line of treatment, and smoking using a linear mixed-effect model. Factors affecting weight over time for the cohort were studied using another linear mixed model adjusting for the above factors along with baseline weight and all drugs received.
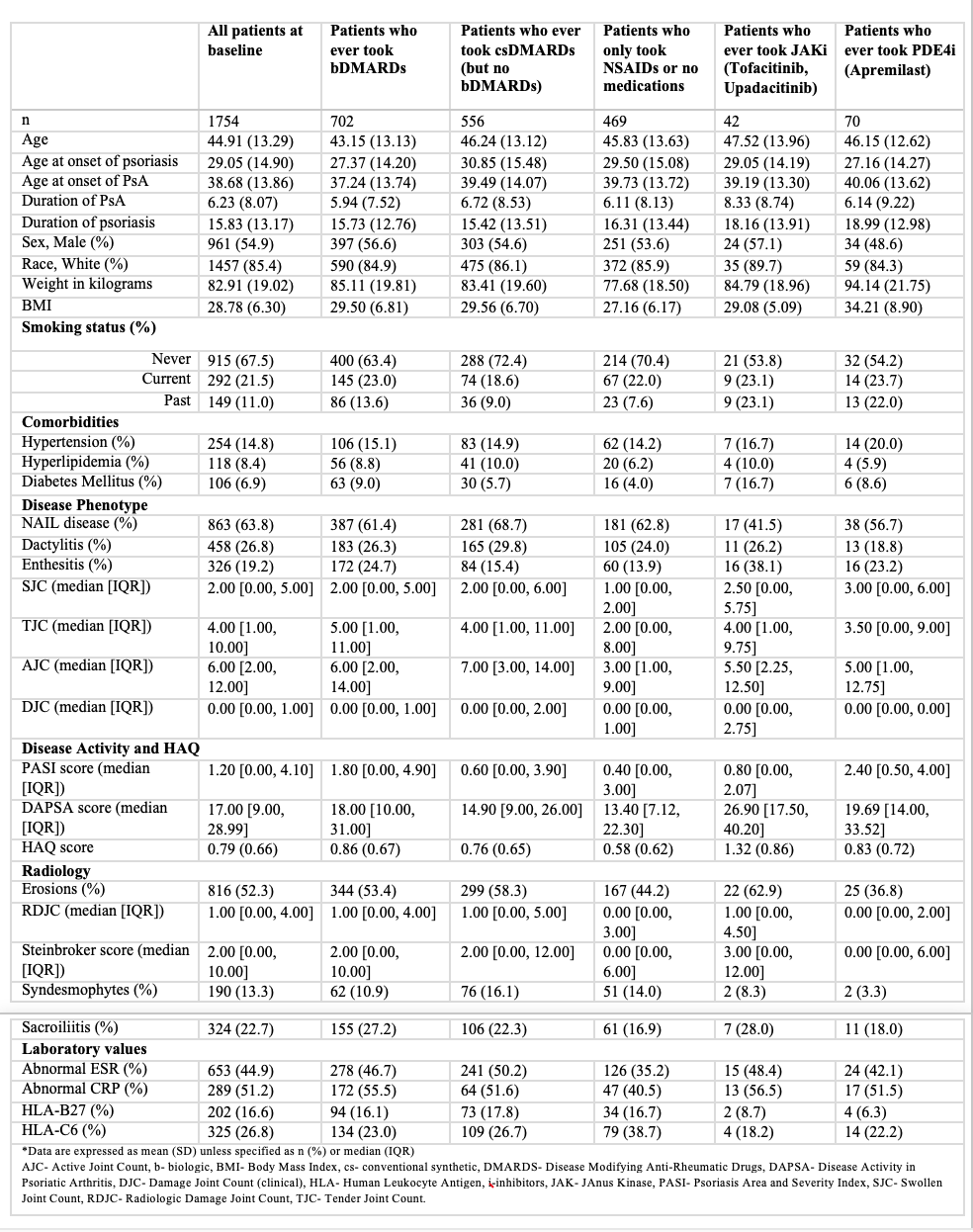
Results: A total of 1754 patients were included, with 473 on NSAIDs or no medications, 571 on csDMARDs, 702 on bDMARDs, 42 on JAKi, and 70 on apremilast. The age at baseline, onset of psoriasis, and PsA were lower in the bDMARD group. Baseline weight, proportion with hypertension, PASI, and DAPSA scores were higher in the apremilast group. The proportion with HLA-C6 was the highest in the NSAIDs or no medication group. (Table 1)
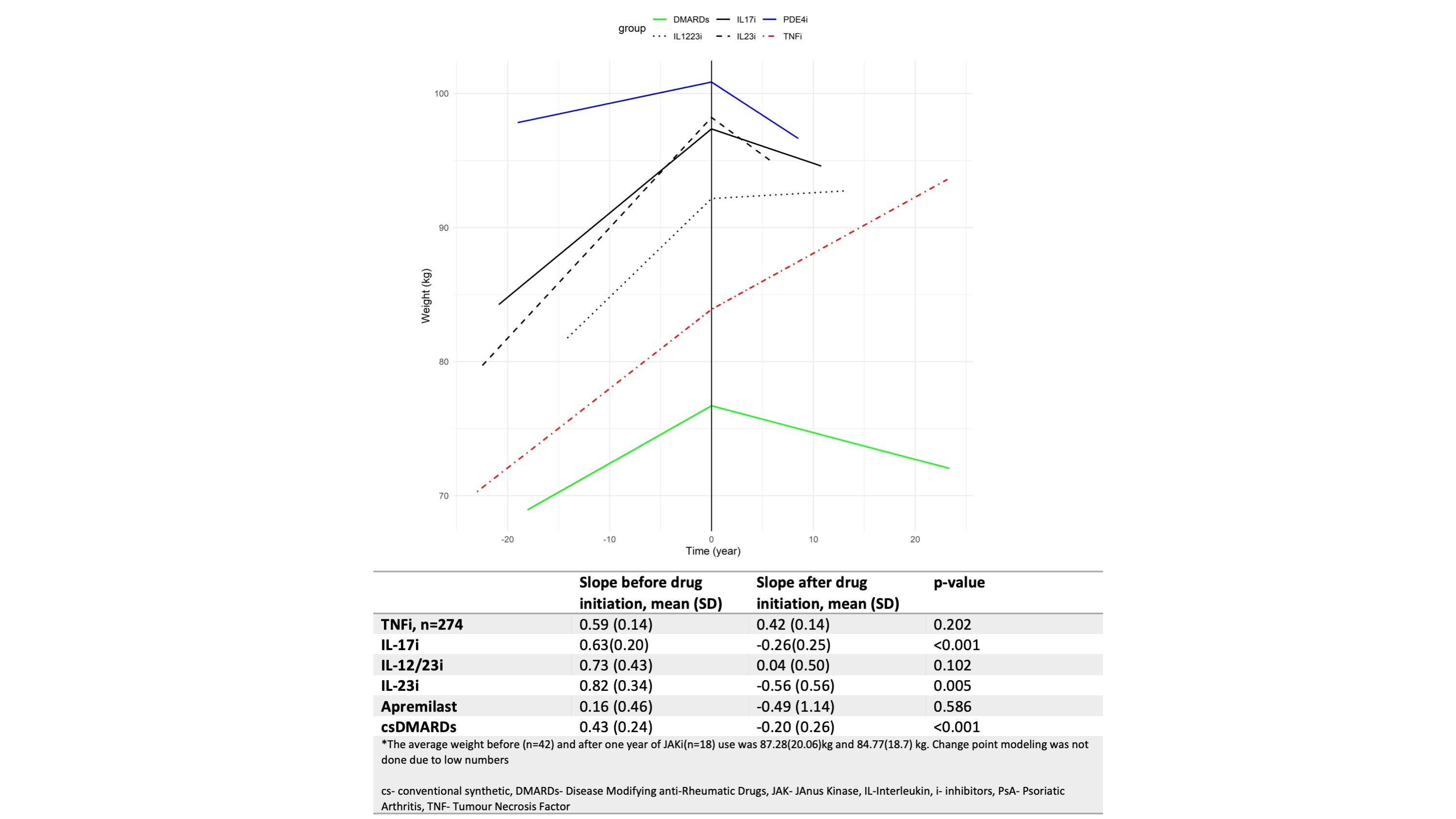
On change point analysis comparing the mean of slopes for weight change before and after medication use for each patient, significant weight loss was observed with IL17i (p 0.001), IL23i (p=0.002), and csDMARDs (p 0.001). The weight change was not significant with TNFi, IL12/23i, or apremilast. However, a trend towards weight gain was observed with TNFi and weight loss with apremilast. (Figure 1)
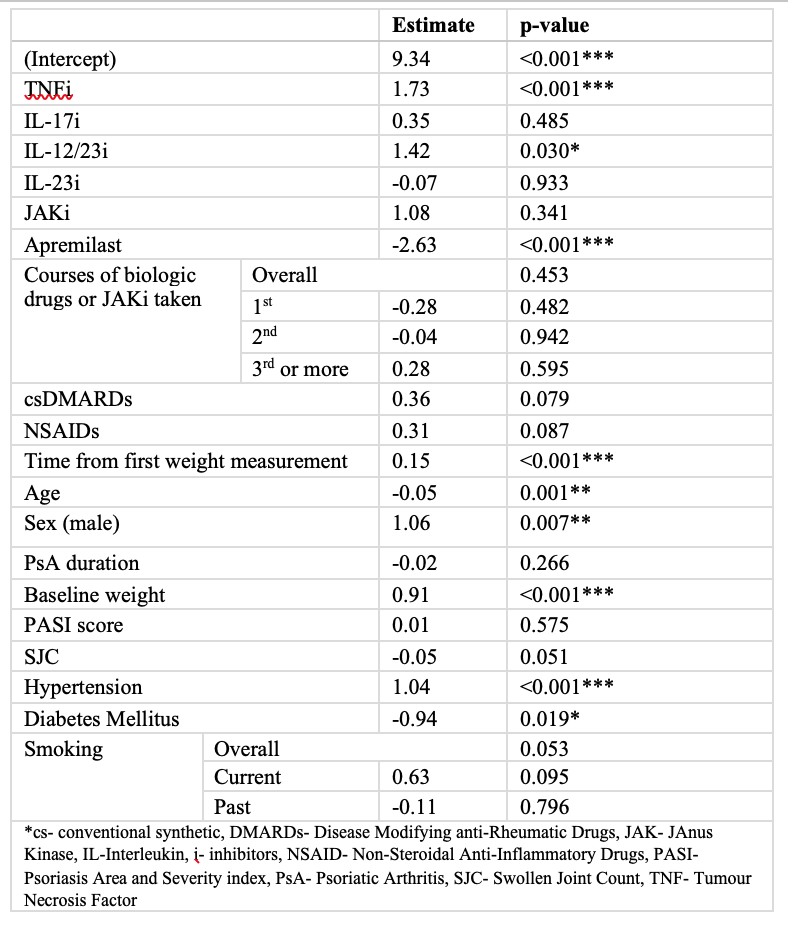
When factors influencing weight were compared across the cohort, weight gain was observed with TNFi, IL-12/23i, longer duration of follow-up, higher baseline weight, hypertension, and male sex. The use of apremilast, older age, and diabetes mellitus were associated with weight loss. (Table 2)
Conclusion: Significant weight loss was observed after initiation of IL17i, IL23i, and csDMARDs in PsA as compared to weight before initiation of these drugs. However, amongst the drugs, the use of TNFi and IL12/23i was associated with weight gain, whereas apremilast was associated with weight loss when compared across the cohort.
To cite this abstract in AMA style:
Mehta P, Kharouf F, Gao S, Gladman D, Chandran V. Exploring Weight Trends in Psoriatic Arthritis: Unraveling Effects of Drugs [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/exploring-weight-trends-in-psoriatic-arthritis-unraveling-effects-of-drugs/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-weight-trends-in-psoriatic-arthritis-unraveling-effects-of-drugs/