Session Information
Session Type: Abstract Session
Session Time: 5:00PM-5:50PM
Background/Purpose: While the etiology of neuropsychiatric lupus (NPSLE) is not fully understood, blood brain barrier (BBB) disruption and localized neuroinflammation are potential mechanisms that contribute to disease progression. Lipocalin-2 (LCN2) is a multi-functional acute phase protein known to affect immune cell function, BBB integrity, and glial cell activation. Previous studies in our laboratory demonstrated the amelioration of behavioral deficits in a LCN2 deficient, lupus-prone mouse model, B6.Sle1.Sle3 (Sle1,3), indicating a potential role of LCN2 in NPSLE. At the transcriptional level, we found that LCN2 deficiency restored genes involved in cognitive function that were downregulated in Sle-1,3 mice. Moreover, LCN2 regulated MMP-9 and Plp1, genes involved in blood brain barrier function and glial cell activation in the brain. In the following studies we examined the role of LCN2 in the neuroinflammation present in the well established MRL/lpr lupus prone strain, as well as the potential role of astrocytes in NPSLE
Methods: Astrocytes and neurons from P1 C57BL/6 pups were isolated and co-cultured for 13 days. On day 14, the co-cultures were treated with either LPS at 100 ng/mL, LCN2 at 5 mcg/ml, or PBS. After 8 hours the cells were fixed and stained for neuron and astrocyte markers by immunofluorescence. Astrocyte activation was analyzed by measuring glial fibrillary acid protein (GFAP) expression through mean fluorescence intensity. For the ex vivo experiments, female 10 week old MRL/lpr mice received an intraperitoneal LPS injection to accelerate systemic inflammation. Eight hours post injection, mice were sacrificed, and their brains paraffin embedded for immunofluorescent staining.
Results: To examine astrocyte activation by LCN2 in the in vitro studies, we analyzed astrocyte morphology and GFAP expression in stimulated cells. Measurement of mean fluorescence intensity showed an increase in GFAP expression with LCN2 stimulation that was similar to the increase seen in the positively treated LPS stimulated cells when compared to the control (Fig. 1B). Additionally, astrocyte morphology in LPS and LCN2 treated cultures showed a similar increase in cellular processes as well as total cell volume size, both indicators of an activated astrocyte morphology (Fig. 1A). Next, we examined the brains of MRL/lpr mice. We found co-staining of LCN2 with GFAP, an astrocyte marker, in the hippocampus and cortex in injected MRL/lpr mice. Additionally, LCN2 was localized around brain endothelial vessels (Fig. 2).
Conclusion: These preliminary results begin to shed light on the significant behavioral improvement we saw in previous experiments in LCN2 deficient mice. Our in vitro results suggest that LCN2 can promote astrocyte activation. Moreover, astrocytes may be the primary source of LCN2 in the brain of MRL/lpr mice. Increased astrocyte LCN2 expression in vivo was mainly localized to the cortex and hippocampus, brain regions that are involved in cognitive function and emotional behaviors, respectively. Our findings of astrocyte activation by LCN2 and the colocalization of LCN2 and astrocytes suggest astrocyte derived LCN2 acting in an autocrine manner to promote pathological neuroinflammation in this NPSLE model.
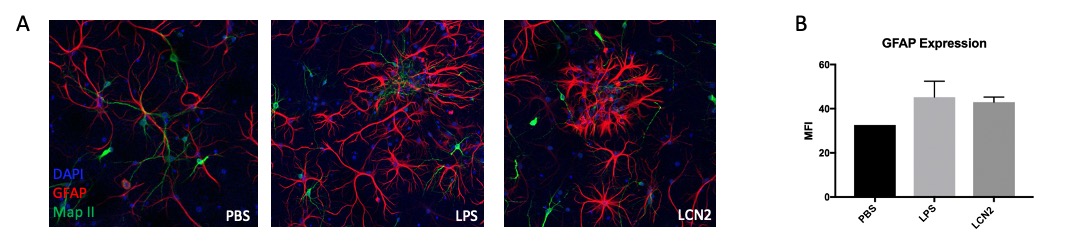 Figure 1: Immunofluorescent staining of neuron-astrocyte co-cultures stimulated with LCN2, LPS, and PBS revealed that LCN2 stimulation leads to activated astrocyte morphology. We observed increased cell volume and cell process extension, indicating higher levels of astrocyte activation in the LPS treated positive control and in the LCN2 experimental group (1A). GFAP mean fluorescence intensity (MFI) was measured using ImageJ software (1B). Green = MAP-II (neurons), Red = GFAP(astrocytes), Blue = DAPI.
Figure 1: Immunofluorescent staining of neuron-astrocyte co-cultures stimulated with LCN2, LPS, and PBS revealed that LCN2 stimulation leads to activated astrocyte morphology. We observed increased cell volume and cell process extension, indicating higher levels of astrocyte activation in the LPS treated positive control and in the LCN2 experimental group (1A). GFAP mean fluorescence intensity (MFI) was measured using ImageJ software (1B). Green = MAP-II (neurons), Red = GFAP(astrocytes), Blue = DAPI.
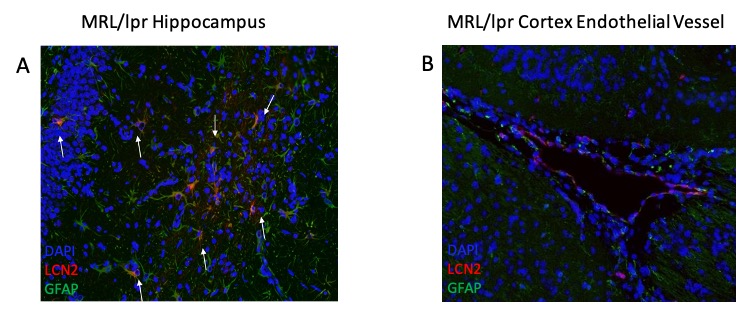 Figure 2: Immunofluorescent staining of MRL/lpr cortex and hippocampus reveals astrocyte-LCN2 co-staining (indicated by white arrows) in MRL/lpr mice injected with LPS, suggesting that under inflammatory stimulus, astrocytes are the main source of LCN2 in MRL/lpr brains. We observed co-staining of astrocytes with LCN2 in the hippocampus (2A) and localization of LCN2 and astrocytes around cortical endothelial vessels (2B). Green = GFAP (astrocytes), Red = LCN2, Blue = DAPI.
Figure 2: Immunofluorescent staining of MRL/lpr cortex and hippocampus reveals astrocyte-LCN2 co-staining (indicated by white arrows) in MRL/lpr mice injected with LPS, suggesting that under inflammatory stimulus, astrocytes are the main source of LCN2 in MRL/lpr brains. We observed co-staining of astrocytes with LCN2 in the hippocampus (2A) and localization of LCN2 and astrocytes around cortical endothelial vessels (2B). Green = GFAP (astrocytes), Red = LCN2, Blue = DAPI.
To cite this abstract in AMA style:
Putterman C, Mike E, Garcia S. Exploring the Role of Lipocalin-2 in Neuropsychiatric SLE Pathogenesis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/exploring-the-role-of-lipocalin-2-in-neuropsychiatric-sle-pathogenesis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-the-role-of-lipocalin-2-in-neuropsychiatric-sle-pathogenesis/
