Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Apremilast is the one targeted immune modulating (TIM) treatment for psoriatic arthritis (PsA) that may be combined with csDMARDs, biologic therapies, or used as a monotherapy. Here we describe the utilization of and experience with apremilast in a large network of community rheumatologists.
Methods: The ARN-TRIO Rheumatology registry consists of EMR (fielded and open text), lab, procedure, infusion, medical claims, and specialty pharmacy data generated in care of >75,000 patients by ARN, a network of independent practices with >200 rheumatologists across the US. For this study, registry data were limited to patients diagnosed with PsA who initiated apremilast or TNF inhibitors between Jan 2014 to Nov 2019 with ≥6 months follow-up. Disease Assessment Scores (DAS) were calculated using CDAI or RAPID3 and analyzed categorically using 4 grade scale. Comparisons between groups used Fisher’s Exact Test for categorical variables. As a surrogate for clinical effectiveness, we examined time from apremilast monotherapy initiation to modification or discontinuation, defined as either drug discontinuation or addition of a csDMARD or TIM drug. Time to event analyses were conducted via KM curves and associated Log-Rank Test for difference in Hazard.
Results: Of the 581 apremilast-treated patients in the study, 325 (56%) had no prior observed PsA treatment, 129 (22%) previously were treated with csDMARDs but not TIM drugs, and 127 (22%) had previously received TIM therapies. Two or more of the apremilast treatment groups were significantly different for gender, race, age, payer, and prior therapies; groups were not different by baseline disease severity. [Table 1] Across these groups, 227 (48%) received apremilast as a monotherapy without prior TIM therapies. A comparator group of 1662 patients who received a TNF inhibitor (TNFi) as a monotherapy and without prior TIM was identified. Characteristics for the apremilast monotherapy and TNFi monotherapy groups differed significantly for gender and race but not for baseline disease severity. [Table 2] Median time from monotherapy start to modification or discontinuation was significantly different between apremilast (19.3 months) and TNFi (30.4 months, p=0.018). In subset analyses, differences in time to modification persisted between apremilast and TNFi monotherapy groups among males (p = 0.037) and patients with severe baseline DAS (p=0.009), but not among females or non-severe baseline DAS. [Figure 1]
Conclusion: The use of apremilast in US community practice is predominantly as a monotherapy. When examined as a first TIM monotherapy, time to modification or discontinuation was significantly less than comparator TNFi monotherapy-treated group. However, when stratified by gender and baseline DAS, female patients and those with low DAS had no difference in time to modification between apremilast and TNFi. These results should be viewed in light of the limitations of the study, which include potential prior treatments or other confounding variables not present in the collected data.
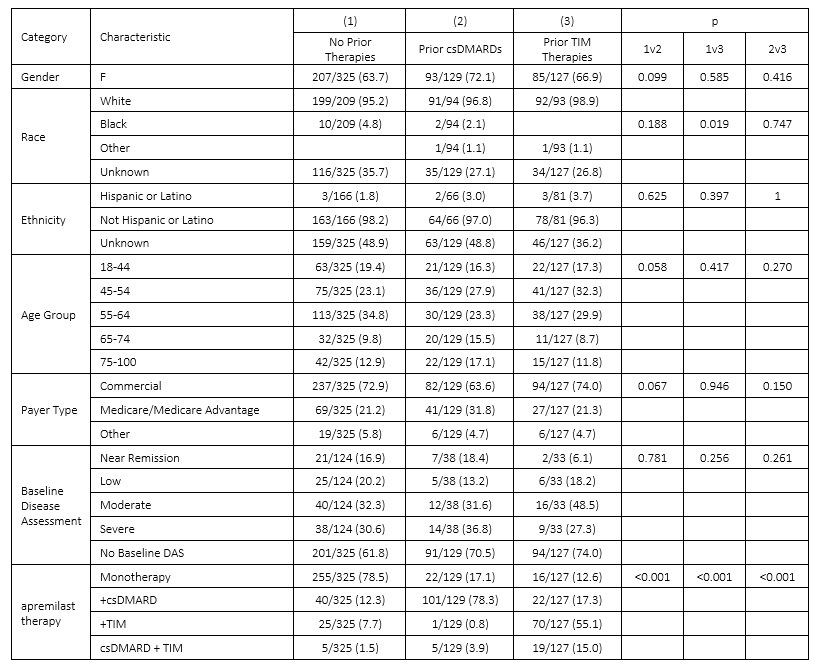 Table 1: Patient Characteristics for apremilast-treated groups
Table 1: Patient Characteristics for apremilast-treated groups
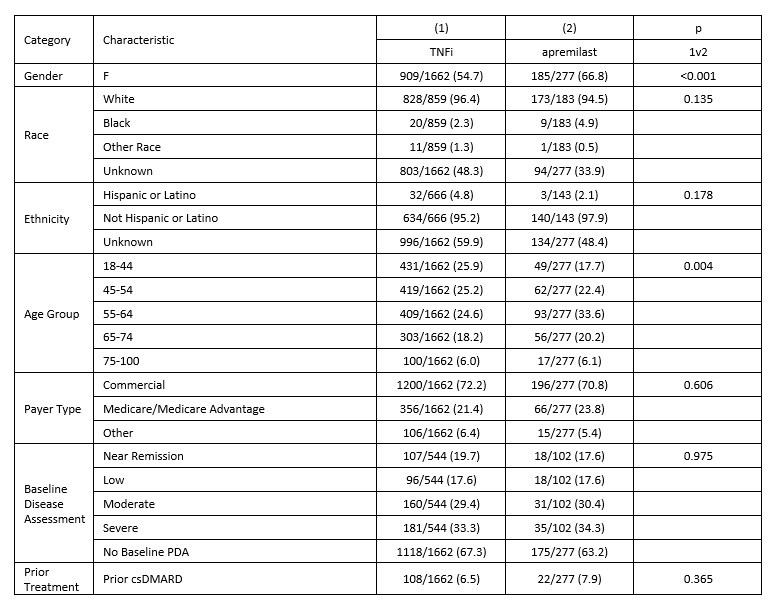 Table 2: Patient Characteristics for patients with qualifying first TIM monotherapy with apremilast or TNFi
Table 2: Patient Characteristics for patients with qualifying first TIM monotherapy with apremilast or TNFi
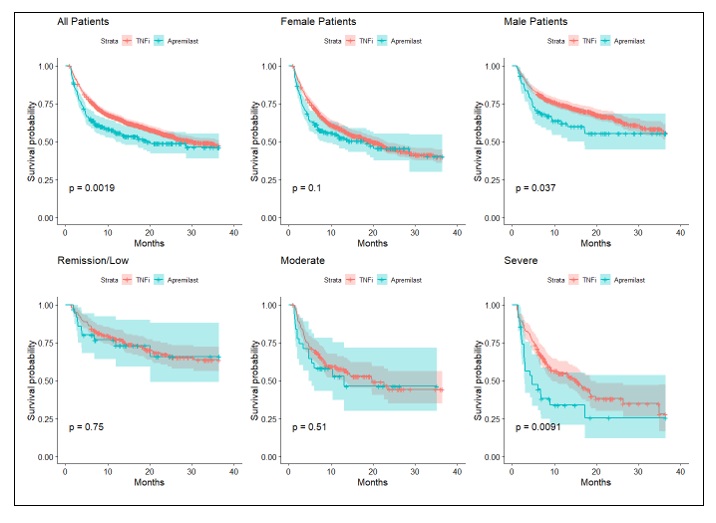 Figure 1: Time to modification or discontinuation of apremilast vs TNFi monotherapies given as first TIM
Figure 1: Time to modification or discontinuation of apremilast vs TNFi monotherapies given as first TIM
To cite this abstract in AMA style:
Edgerton C, Frick A, Helfgott S, Huston K, Singh J, Soloman N. Experience with Apremilast in Treatment of Psoriatic Arthritis in US Clinical Practice; Assessments from Trio Health and the American Rheumatology Network (ARN) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/experience-with-apremilast-in-treatment-of-psoriatic-arthritis-in-us-clinical-practice-assessments-from-trio-health-and-the-american-rheumatology-network-arn/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/experience-with-apremilast-in-treatment-of-psoriatic-arthritis-in-us-clinical-practice-assessments-from-trio-health-and-the-american-rheumatology-network-arn/
