Session Information
Date: Monday, November 18, 2024
Title: Muscle Biology, Myositis & Myopathies – Basic & Clinical Science Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Idiopathic inflammatory myopathy (IIM) is a rare multisystem inflammatory condition leading to significant disability. The management of IIM remains challenging. B cell targeting therapies are used in refractory cases. Although there were no significant differences between study arms in large randomized, double-blind, placebo-phase trial with rituximab in IIM, 83% of refractory patients improved, suggesting the importance of B cells in the pathogenesis of IIM.
Obinutuzumab was reported for use in autoimmune conditions in those with rituximab allergies. Furthermore, Obinutuzumab has higher B cell depletion potency than rituximab. To date, there is only one reported case of Obinutuzumab use in IIM with rituximab refractory Antisynthetase Syndrome (ASyS) that had marked improvement after Obinutuzumab. We report our experience at the Myositis Northwell Center with the use of Obinutuzumab in patients with IIM.
Methods: The Northwell database was searched for Obinutuzumab infusion code and the diagnosis codes of IIM (dermatomyositis, polymyositis, ASyS, including overlap myositis). 6 patients were identified. 1 patient was excluded because Obinutuzumab was ordered but the patient never received it. A retrospective analysis was performed on patients meeting 2017 EULAR/ACR classification criteria for IIM. Demographic, clinical, and serological data were collected, along with PFTs and CT chests. Physician global score was used to assess response to Obinutuzumab. Descriptive statistics were used.
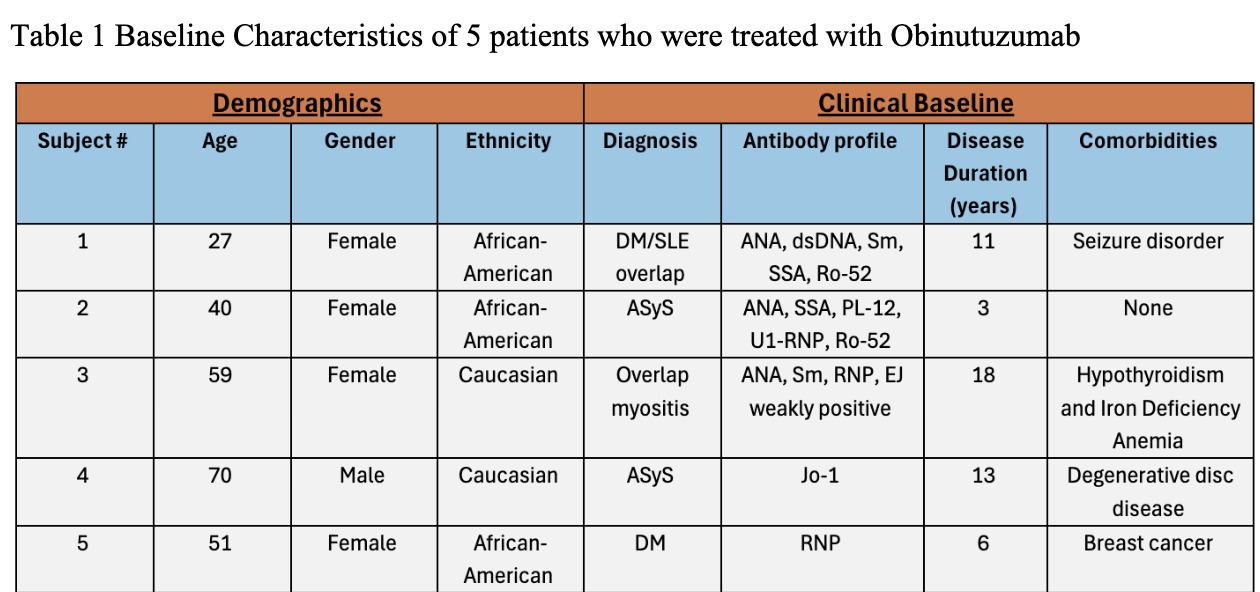
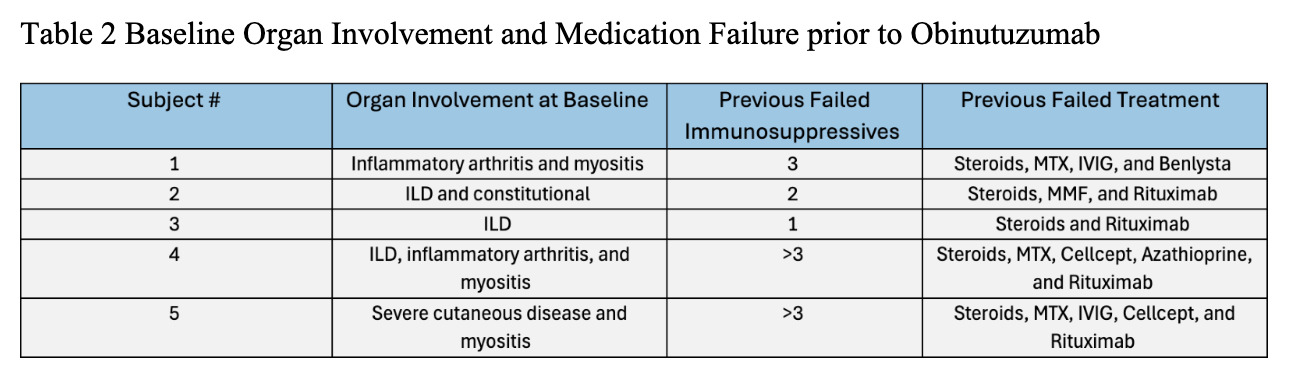
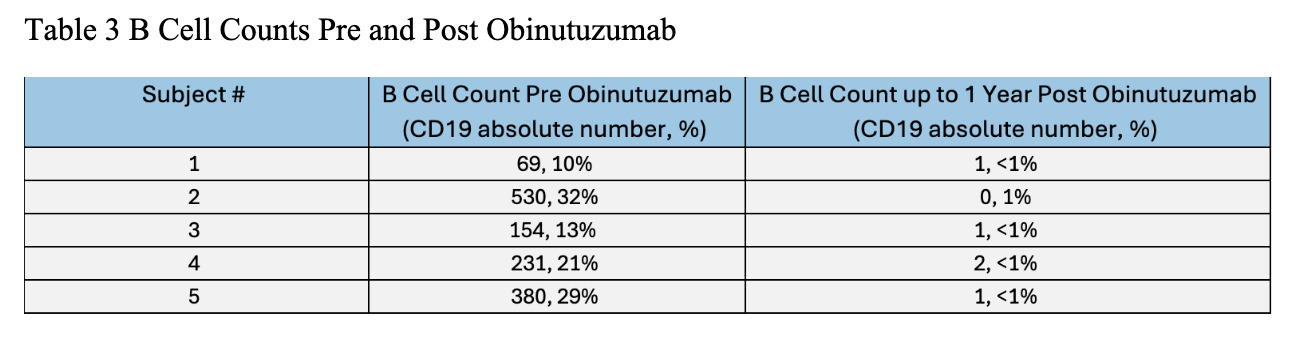
Results: Demographic and clinical characteristics of 5 patients are noted in Table 1. Of five patients, 2 had ASyS, 1 dermatomyositis, and 2 overlap myositis with average disease duration 10 years. Most patients were female and African American. Most failed at least 3 immunosuppressive agents; 1 failed rituximab. The reason for Obinutuzumab start was allergy to rituximab in 4 patients, worsening of interstitial lung disease and myositis activity in 3, inflammatory arthritis in 2, and severe cutaneous disease in 1 (Table 2). Four patients had >1 year follow up after Obinutuzumab with maximum follow-up of 4 years. One received the drug two months prior to analysis. Clinical improvement was noted at 3-6 months post Obinutuzumab in skin, muscle, lung, and joint domains. Prior elevated CPK normalized after treatment. PFTs and CT chest remained stable in 3 patients despite symptomatic improvement. All patients had pre and post B cell counts checked (Table 3). Most depleted B cells within 1-3 months and repleted after 1 year. Only 1 patient required redosing of Obinutuzumab which was decided based on incomplete clinical response and B cell repopulation. Patients were able to discontinue steroids in 3 cases and 1 patient stopped Methotrexate after Obinutuzumab. On the last observation all patients were deemed to be responders based on physician global assessment. No one developed hypogammaglobulinemia post Obinutuzumab, and there were no significant infections or hospitalizations.
Conclusion: This is the first case series of successful and safe use of Obinutuzumab in IIM. This provides a signal to design a large-scale clinical trial with Obinutuzumab in IIM.
To cite this abstract in AMA style:
Ilic I, Marder G. Experience on the Use of Obinutuzumab off Label in Patients with Refractory Idiopathic Inflammatory Myopathy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/experience-on-the-use-of-obinutuzumab-off-label-in-patients-with-refractory-idiopathic-inflammatory-myopathy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/experience-on-the-use-of-obinutuzumab-off-label-in-patients-with-refractory-idiopathic-inflammatory-myopathy/