Session Information
Date: Monday, November 18, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Takayasu’s arteritis (TAK) is a rare inflammatory disease primarily involving the aorta and its major branches. Multiple genetic association studies on common variants in TAK have led to progress in understanding the mechanisms of TAK. However, the contribution of rare variants to TAK is still poorly understood.
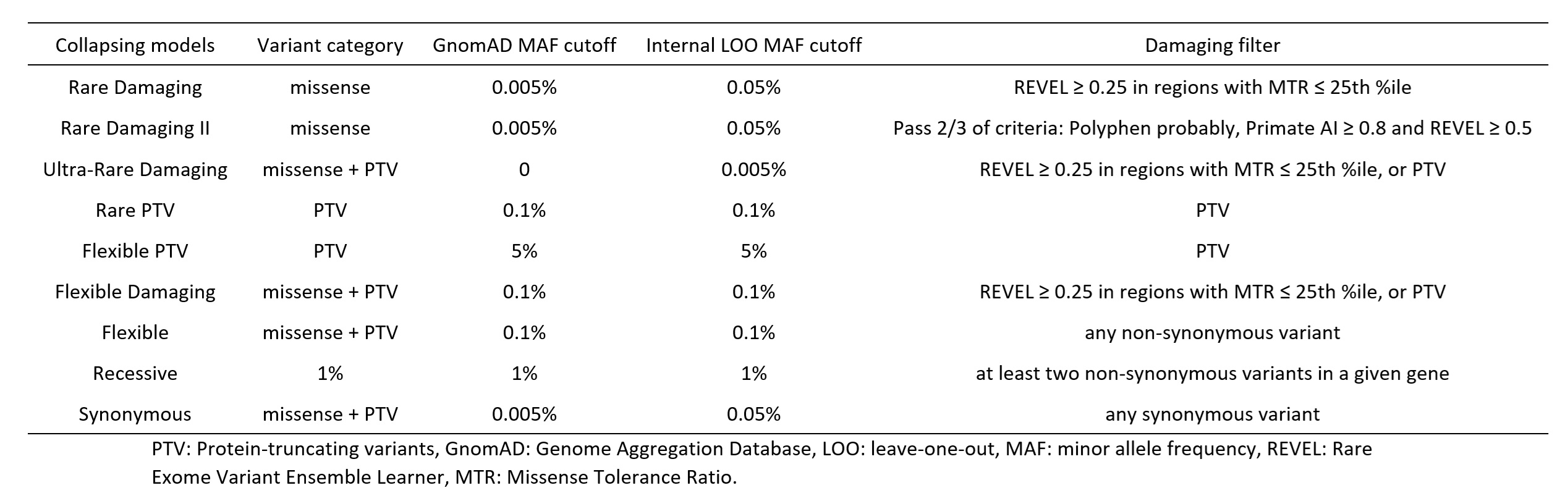
Methods: This is a case-control exome-wide rare variant association study (RVAS) in a multi-ancestral population. Exome controls were selected from among individuals who did not have a known autoimmune phenotype. Multiple quality control steps were implemented to select high-quality exonic variants with balanced coverage between cases and controls. Principal component analysis (PCA) and kinship pruning were performed to include only unrelated individuals. PCA-based clustering analysis was performed to generate ancestry clusters. Nine collapsing models were defined based on population allele frequency and predicted deleterious effects (Table 1). Gene-level collapsing analysis was performed using Cochran-Mantel-Haenszel test stratified by the ancestry cluster membership.
Results: After quality control, the study included data from 206 patients with TAK and 11,957 controls. Participants were clustered into seven group based on their ancestral background (“European 1”, “European 2”, “African and Latinx”, “Latinx”, “East Asian”, “South Asian”, and “Middle Eastern”). No gene reached exome-wide statistical significance in any of the collapsing models. In the Flexible Model, IL15 was top-ranked (2.5% vs 0.2%, odds ratio (OR) = 14, p = 8.3 x 10-5). In the Rare Protein-Truncating Variant (PTV) Model, there was a numerically higher burden of PTVs in NFKB1 (1.0% vs 0.009%, OR = 91, p = 0.002). Two patients with TAK had a frameshift (P444fs) and stop-gained (W295X) variant in NFKB1, respectively. These two NFKB1 variants are pathogenic for haploinsufficiency of the Nuclear Factor Kappa B Subunit 1 (NF-kappaB1) p50 subunit based on the American College of Medical Genetics and Genomics guideline.
Conclusion: Our RVAS did not find statistically significant genes associated with TAK, but was limited by a small sample size of the case cohort. A numerically higher burden of rare variants in IL15, a pro-inflammatory cytokine that regulates the activation of T and Natural Killer cells, were observed in TAK. We have also found two individuals with TAK who had a genetic diagnosis of haploinsufficiency of the NF-kappaB1 p50 subunit, whose phenotypes have been reported to involve a broad spectrum of immunodeficiency, autoimmunity, autoinflammation, and cancer. Our study supports TAK as likely being an inflammatory manifestation of the NFKB1-related monogenic phenotypes.
To cite this abstract in AMA style:
Luo Y, deng Z, Quinn K, Sikora K, Kastner D, Kiryluk K, Sawalha A, Merkel P, Grayson P. Exome-Wide Rare Variant Association Study of Takayasu’s Arteritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/exome-wide-rare-variant-association-study-of-takayasus-arteritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exome-wide-rare-variant-association-study-of-takayasus-arteritis/