Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Over a third of RA patients are managed with long-term oral glucocorticoids (GC), defined as daily GC use for ≥3 months[1]. Due to dose-dependent toxicity concerns, current ACR guidelines for RA management recommend tapering GC when possible[2]. Despite this, GC tapering is under-studied in RA. This is particularly true among patients with established RA ( >2 years’ duration) who use long-term GC. These patients often have high cumulative GC exposure levels and GC-associated comorbidities, and are prone to withdrawal symptoms when GC are tapered. Our aim was to review existing clinical trials that evaluate GC tapering in RA.
Methods: We searched clinicaltrials.gov for registered studies of oral GC use in RA patients since 2008 (Fig. 1). In addition, we reviewed studies captured by a previous systematic review of clinical trials of GC tapering in RA, which included years 1972 to 2011 [3].
Results: Results: 2300 entries from clinicaltrials.gov were screened (Fig. 1-2). 179 entries evaluated GC, and 11 evaluated oral GC tapering. Five additional studies were identified from the previous systematic review, for a total of 16 studies. Of the 11 clinicaltrials.gov entries, seven incorporated GC tapers as induction treatment or bridge therapy for early RA patients (Table 1). Two entries did not specify starting GC dose or taper duration. One entry (NCT02573012) compared RA disease activity among patients on tocilizumab randomized to continue vs. stop vs taper off long-term oral GC. The last (NCT02997605) compared efficacy of prednisone vs. hydrocortisone tapers in allowing RA patients to discontinue long-term oral GC. Of the five studies identified from the previous systematic review, three incorporated GC tapers as induction treatment for early RA patients. The remaining two studies compared RA disease activity (Tengstrand et al) or radiographic progression (Pincus et al) among patients randomized to continue vs taper off long-term GC. Of the 16 studies reviewed, only one (NCT02997605) evaluated GC withdrawal symptoms as a secondary outcome. Another (Tengstrand et al) allowed for slower GC taper if “withdrawal symptoms” occurred, but did not specify how these were defined.
Conclusion: There is little published trial data to guide clinicians attempting to taper long-term GC among patients with established RA. We identified only one trial directly comparing two GC tapering regimens, which is currently unpublished. Further work is needed to develop data-driven protocols for GC tapering that account for GC withdrawal symptoms.
- Wallace B, Lin P, Kamdar N, Noureldin M, Hayward R, Fox DA, et al. Patterns of Glucocorticoid Use and Provider-Level Variation in a Commercially Insured Incident Rheumatoid Arthritis Population [abstract]. Arthritis & rheumatology. 2018; 70(Suppl. 10).
- Singh JA, Saag KG, Bridges SL, Jr., Akl EA, Bannuru RR, Sullivan MC, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis & rheumatology. 2016 Jan; 68(1):1-26.
- Volkmann ER, Rezai S, Tarp S, Woodworth TG, Furst DE. We still don’t know how to taper glucocorticoids in rheumatoid arthritis, and we can do better. The Journal of rheumatology. 2013 Oct; 40(10):1646-1649.
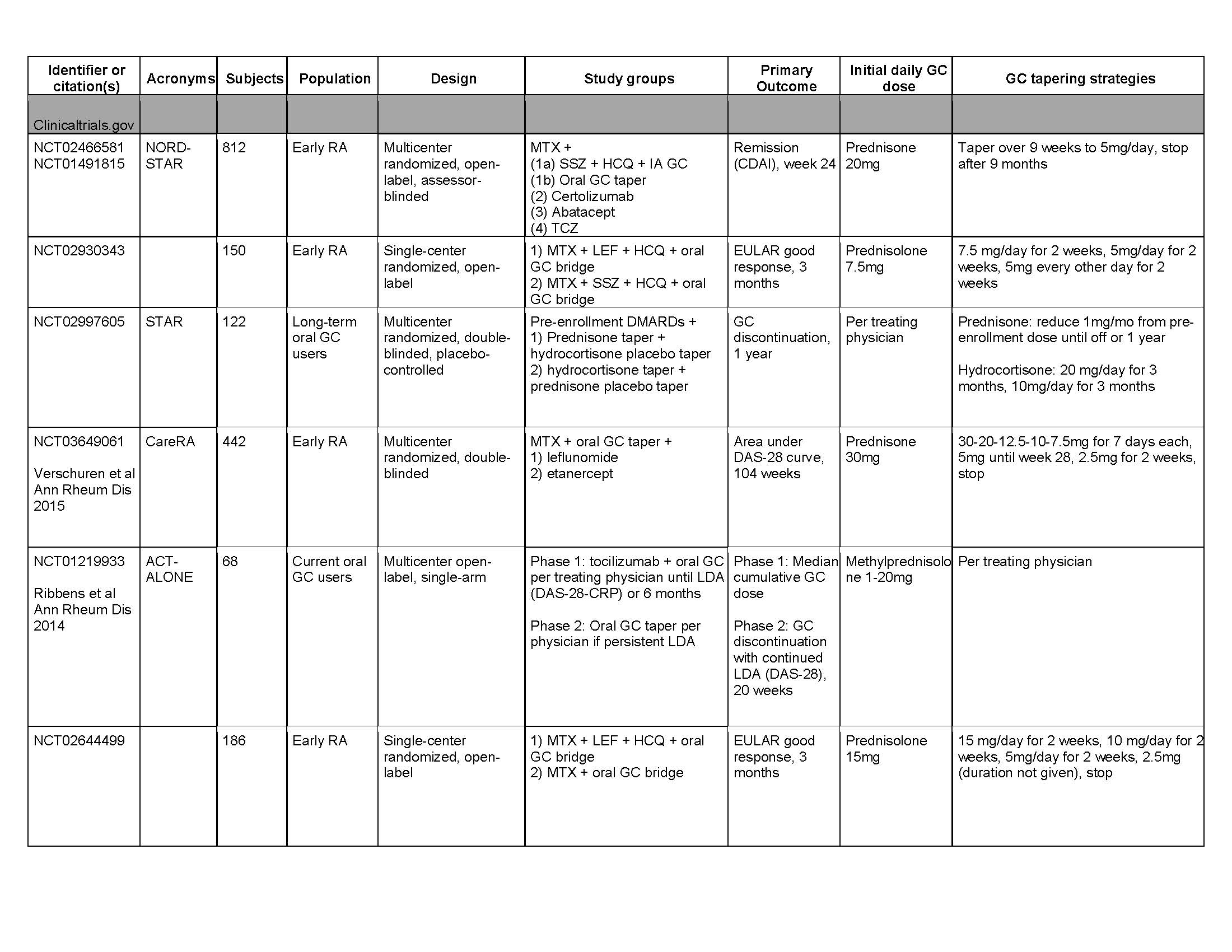
table 1 acr abstract clinicaltrials.gov
To cite this abstract in AMA style:
Wallace B, Wallace D, Waljee A, Clauw D. Evidence to Guide Glucocorticoid Tapering Is Lacking in RA [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evidence-to-guide-glucocorticoid-tapering-is-lacking-in-ra/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evidence-to-guide-glucocorticoid-tapering-is-lacking-in-ra/


