Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: To assess whether two different biological therapies (BT), tumor necrosis factor inhibitor (TNFi) and ritixumab (RTX), are related to a different course and severity of SARS-CoV-2 infection in patients with rheumatic diseases.
Methods: Observational and retrospective multicenter study that includes patients under follow-up by the rheumatology services of Hospital La Mancha Centro, Hospital Nª Sª del Prado and Hospital de Tomelloso, and who received some BT from at least 3 months prior to the beginning of the COVID19 pandemic until March 2021. Sociodemographic, clinical and treatment variables were collected through digital medical history, as well as the presence of confirmed SARS-CoV-2 infection (by nasopharyngeal RT-PCR, rapid antigen or antibodies IgG tests) and its posterior evolution. The development of pneumonia and the need for hospital admission, the need for ventilatory support, refractoriness to corticosteroids (CS), absence of development of IgG antibodies against the virus, higher WHO clinical progression scale and the exitus were considered worse evolution and greater severity. These evolution variables were compared between the patients who received TNFi versus others and also between who received RTX versus others.
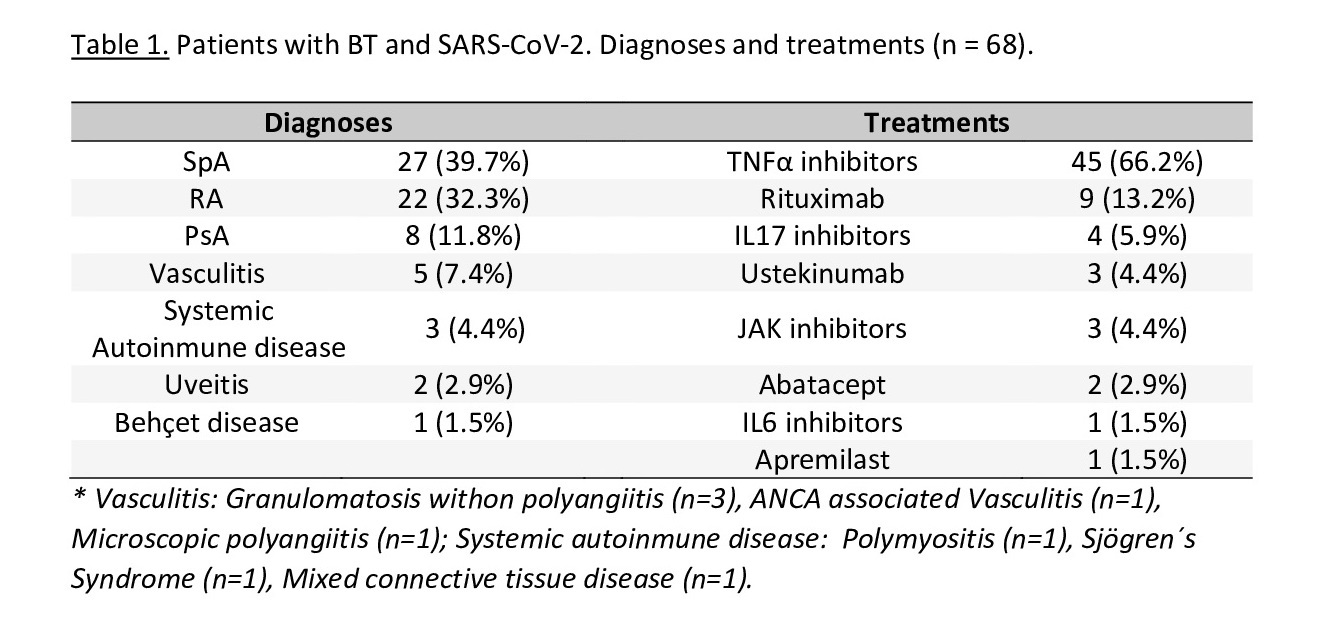
Results: In the 3 hospitals, a total of 372 patients with rheumatic diseases received BT during follow-up, of which 68 patients (18.3%) had SARS-CoV-2 infection, with a mean age of 54.8±13.8 years (54.4% women and 45.6% men). The most frequent diagnoses were inflammatory spondyloarthropathy (39.7%), rheumatoid arthritis (32.3%) and psoriatic arthritis (11.8%), and the BT administered were TNFi (66.2%), RTX (13.2%) and IL17 inhibitors (5.9%) (table 1). The mean time of BT administration was 54.9±46.4 months.
Of the patients infected with SARS-CoV-2, 21 (30.9%) developed pneumonia, 17 (25%) suffered hospital admission and 34 (50%) required some treatment (of which 9 (13.2%) were initially refractory to CS treatment and 6 (8.8%) required ventilatory support). The mean time of hospitalization was 25.1±30.5 days and the mean of the WHO clinical progression scale was 2.8±2.1, with 2 dead patients (2.9%). At the end of the follow-up, 9 patients (13.2%) had not developed IgG antibodies against the virus.
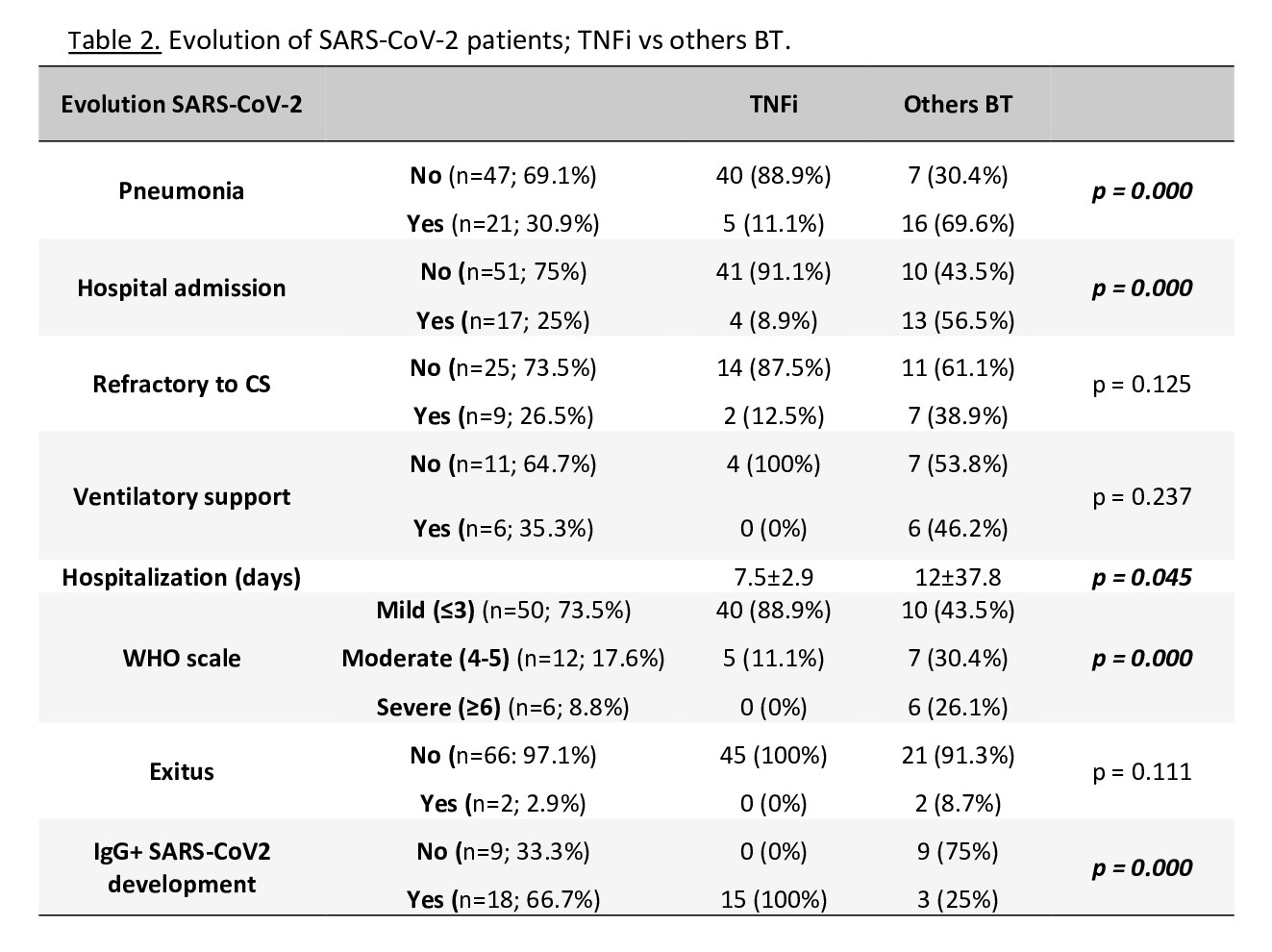
In the group TNFi compared to the others TB, there was a lower percentage of patients who developed pneumonia and hospitalization, had a lower score on the WHO clinical progresión scale and a shorter hospitalization time, with all patients developing SARS-CoV-2 IgG after infection. No patient in this group died (table 2).
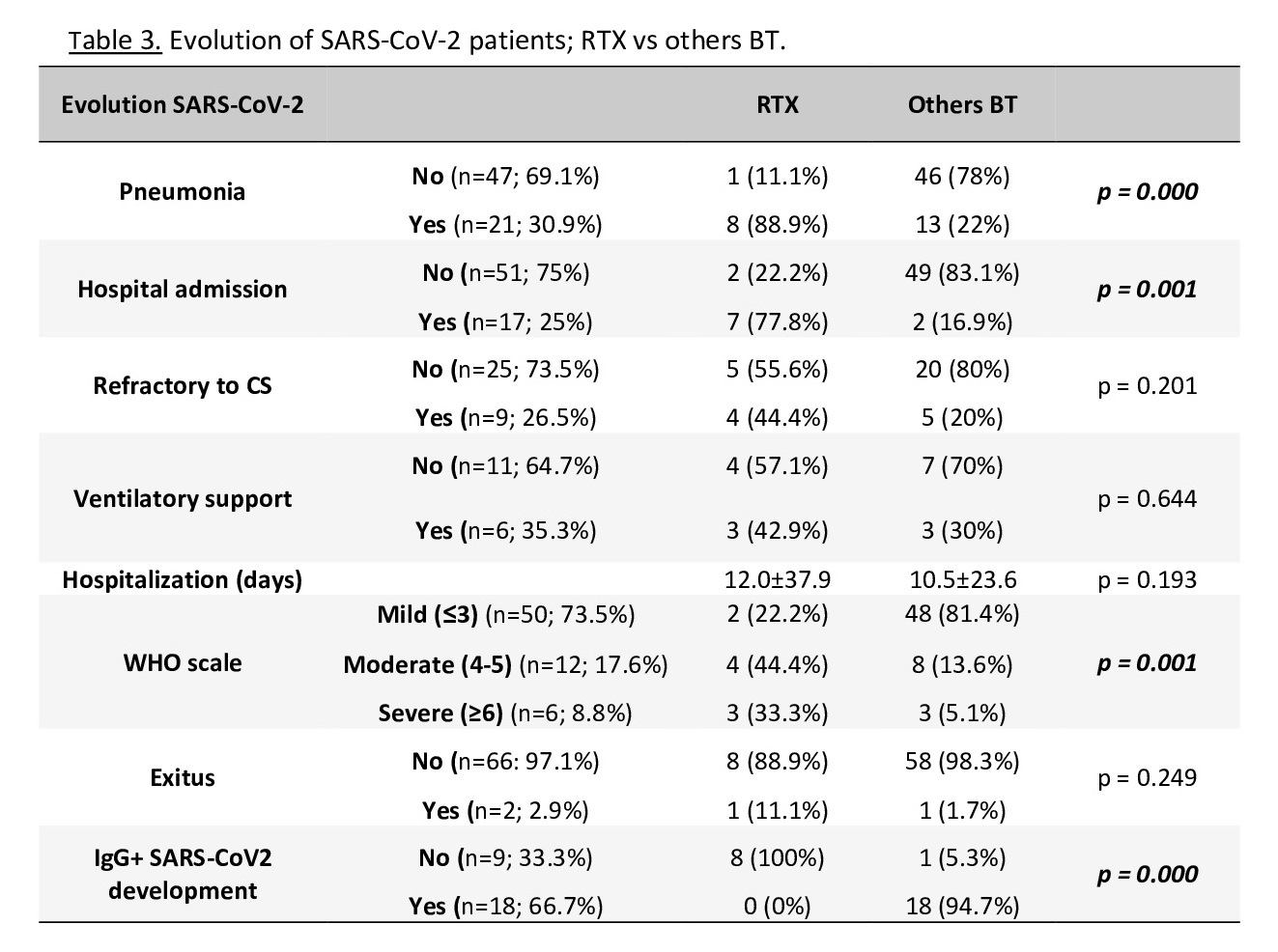
In the RTX group there was a higher percentage of SARS-CoV-2 infection (37.5%) than in others (17%); also, the patients with RTX had a higher percentage of development of pneumonia and hospital admission and had a higher score on the WHO scale, with no developing of SARS-CoV-2 IgG during follow-up in any of them. One patient in this group died (table 3).
Conclusion: In our rheumatic patients under treatment with TB there has been a different evolution of the SARS-CoV-2 infection, having been better and less severe in patients with TNFi and worse and more severe in patients with RTX.
To cite this abstract in AMA style:
Sánchez-Fernández S, Rojas Vargas L, Del Olmo Pérez L, García Morales P, Alía Jiménez A, Carrasco Fernández J, Masegosa Casanova S. Evaluation of the Possible Different Evolution of SARS-CoV-2 Infection with Tumor Necrosis Factor Inhibitors or with Rituximab in Rheumatic Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-possible-different-evolution-of-sars-cov-2-infection-with-tumor-necrosis-factor-inhibitors-or-with-rituximab-in-rheumatic-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-possible-different-evolution-of-sars-cov-2-infection-with-tumor-necrosis-factor-inhibitors-or-with-rituximab-in-rheumatic-patients/