Session Information
Date: Monday, November 8, 2021
Title: Epidemiology & Public Health Poster III: Other Rheumatic & Musculoskeletal Diseases (1022–1060)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: The Manhattan Lupus Surveillance Program (MLSP) is a multi-racial/ethnic population-based registry with the primary goal to determine the prevalence and incidence of Systemic Lupus Erythematosus (SLE). In this study, we compare the three most commonly used classification criteria for SLE (1997 revised ACR, Systemic Lupus International Collaborating Clinics (SLICC) and the recent EULAR/ACR classification criteria) to identify cases that fulfilled only one of the classification criteria and explore each criteria set’s unique cases. In addition, we used the EULAR/ACR criteria to determine the incidence and prevalence of SLE in Manhattan.
Methods: MLSP cases were identified from Manhattan-based hospitals and rheumatologists, and state population databases. For this analysis, SLE cases were defined as fulfilling 1) the 1997 ACR classification criteria, 2) the SLICC criteria or 3) EULAR/ACR classification criteria. We quantified the number of cases that uniquely associated with each classification criteria and the number that fulfilled all three classifications. Prevalence (2007) and incidence rates (2007-2009) using the EULAR/ACR classification criteria and associated 95% confidence intervals (CI) were calculated using denominators obtained from the US Census data (revised 2000-2009 intercensal population files) for Manhattan.
Results: Overall 1,568 cases fulfilled at least one of the three classification criteria. Of those, 1008 (64.3%) cases fulfilled all three classification criteria, 166 (10.5%) fulfilled only the SLICC criteria, 50 (3.2%) fulfilled only the 1997 ACR criteria and 36 (2.3%) fulfilled the EULAR/ACR criteria with the remaining cases fulfilling a combination of two classification criteria. Cases that only met one of the classification criteria, and the reasons why they did not meet the other two classification criteria with example cases, are detailed in Tables 1-3. Based on the EULAR/ACR classification criteria, the age-adjusted overall prevalence and incidence rates of SLE in Manhattan were 59.8 (n=1,029, 95%CI:56.1- 63.6) and 4.9 (n=245, 95%CI 4.3-5.5) per 100,000 population. Prevalence was 9 times higher and incidence was 6.9 times higher among females compared to males. The age-adjusted prevalence per 100,000 was highest among non-Hispanic Black females (198.9), followed by Hispanic females (133.1), non-Hispanic Asian/Pacific Islander females (97.7) and non-Hispanic White females (59.8). Age-adjusted incidence rates per 100,000 were highest in non-Hispanic Black females (15.8), followed by Hispanic females (7.5), non-Hispanic Asian/Pacific Islander females (7.3) and non-Hispanic White females (6.3). Prevalence and incidence rates for males followed a similar pattern.
Conclusion: Applying the three commonly used classification systems to a multi-racial/ethnic population-based registry allowed for identifying unique cases of SLE who only fulfilled one classification system. The EULAR/ACR classification criteria revealed similar prevalence and incidence estimates and gender and racial/ethnic disparities to the previously published results from the MLSP using the 1997 revised ACR and SLICC classification criteria.
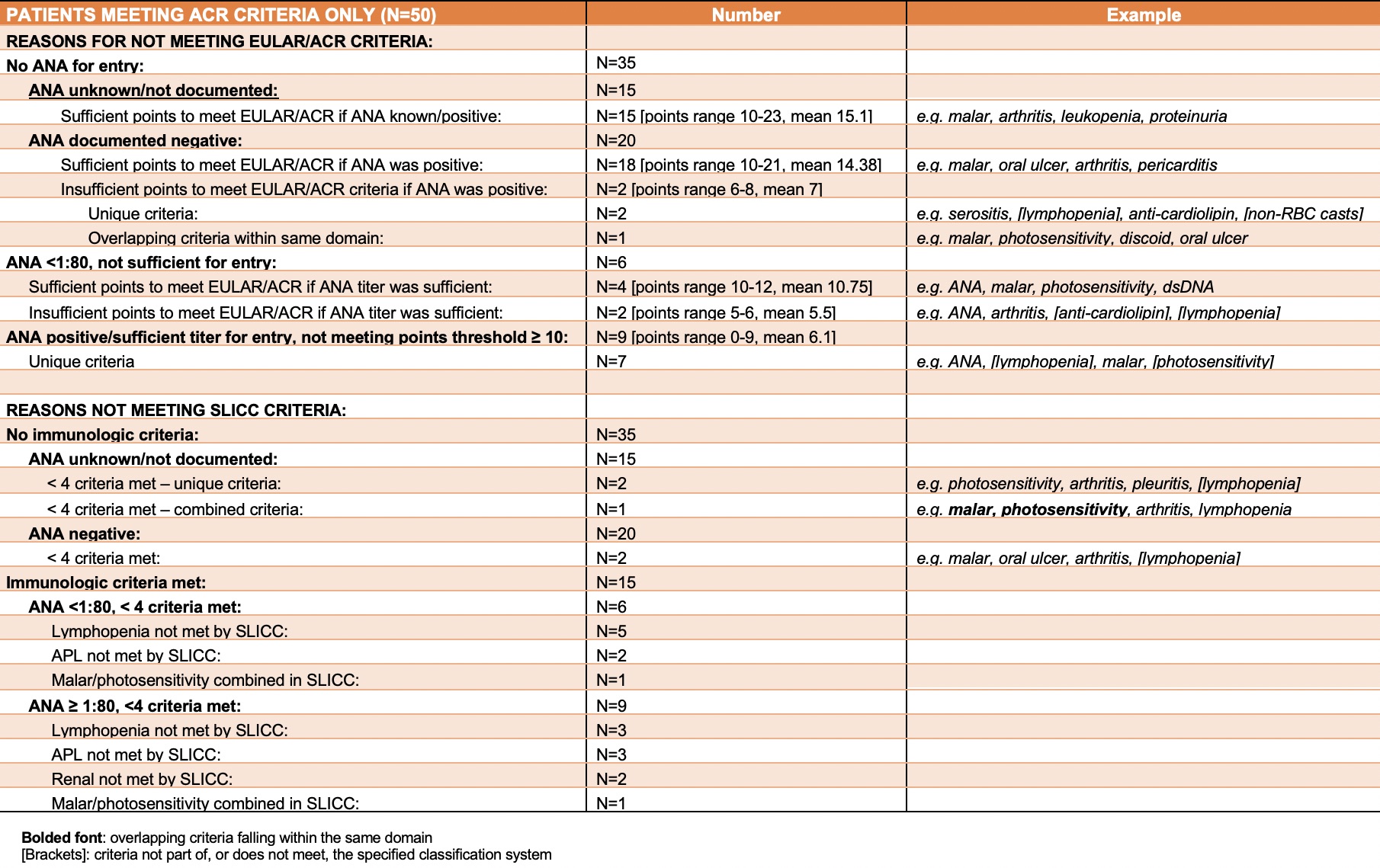 Table 1 – Explanations for patients meeting ACR criteria only
Table 1 – Explanations for patients meeting ACR criteria only
 Table 2 – Explanations for patients meeting SLICC criteria only
Table 2 – Explanations for patients meeting SLICC criteria only
 Table 3 – Explanations for patients meeting EULAR/ACR criteria only
Table 3 – Explanations for patients meeting EULAR/ACR criteria only
To cite this abstract in AMA style:
Guttmann A, Denvir B, Buyon J, Aringer M, Belmont H, Sahl S, Salmon J, Askanase A, Bathon J, Geraldino L, Ali Y, Ginzler E, Putterman C, Gordon C, Helmick C, Parton H, Izmirly P. Evaluation of the EULAR/ACR Classification Criteria for Systemic Lupus Erythematosus in a Population-Based Registry [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/evaluation-of-the-eular-acr-classification-criteria-for-systemic-lupus-erythematosus-in-a-population-based-registry/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-eular-acr-classification-criteria-for-systemic-lupus-erythematosus-in-a-population-based-registry/
