Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Systemic methotrexate (MTX) is the first-line disease-modifying antirheumatic drug (DMARD) for treating early and established rheumatoid arthritis (RA). When compared to oral MTX, subcutaneous MTX enhances tolerability with a significant reduction in gastrointestinal side effects. Subcutaneous MTX has further demonstrated better clinical efficacy than oral MTX in patients who were both oral MTX-experienced and MTX-naïve. This study aimed to better quantify the role of subcutaneous MTX in delaying initiation of biologic DMARD (bioDMARD).
Methods:
In this retrospective cohort analysis, new users of oral MTX were identified between January 1, 2008 and December 31, 2014 (N=42,413). Patients aged 18 years or older with continuous health plan membership, drug benefit, and RA diagnosis (n=7,968) were included in this analysis. Patients who had at least one ≥2.5 mg increase in the weekly oral MTX dose (n=3,970) were compared to those who switched from oral MTX to subcutaneous MTX (n=421). The primary outcome was the likelihood of initiating a bioDMARD, which was analyzed using Cox proportional hazard model. Other outcomes measured were the timing of changes in treatment, and doses of oral or subcutaneous MTX at the time of switches or at the end of follow-up.
Results:
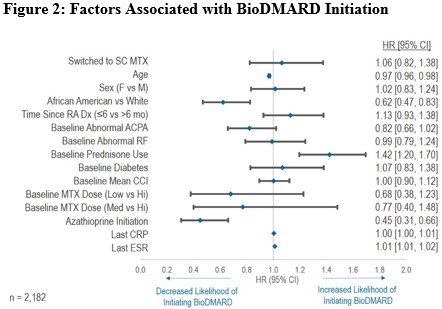
Comparing the two treatment strategies, the unadjusted and adjusted Cox regression analyses showed no significant difference in the likelihood of initiating bioDMARD. Factors associated with a reduced likelihood of initiation of bioDMARD included older age, African American, and the initiation of azathioprine. In contrast, the use of systemic prednisone at baseline and higher erythrocyte sedimentation rate (ESR) during follow-up were associated with an increased likelihood of initiation of bioDMARD. Among the patients who started bioDMARD, more than two-third of the patients never received MTX at doses >20 mg/week prior to initiating bioDMARD. Compared to patients who had a dose increase in oral MTX, patients who switched to subcutaneous MTX significantly delayed the use of bioDMARDs by 9 months (p<0.001).
Conclusion:
There is no significant difference in the likelihood of initiating bioDMARD between the two treatment strategies. Compared to oral MTX group, switching to subcutaneous MTX delayed the use of bioDMARD by 9 months. Given that bioDMARDs are costly and require injections, switching from oral MTX to subcutaneous MTX before using bioDMARD may be a reasonable alternative for patients who fail oral MTX.
To cite this abstract in AMA style:
Wan J, Spence M, Niu F, Hui R, Cheng S, Saito L, Lin A. Evaluation of the Effectiveness of Injectable Methotrexate for the Treatment of Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-the-effectiveness-of-injectable-methotrexate-for-the-treatment-of-rheumatoid-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-effectiveness-of-injectable-methotrexate-for-the-treatment-of-rheumatoid-arthritis/