Background/Purpose: Non-biologic (NB) disease-modifying antirheumatic drugs (DMARD) such as methotrexate (MTX) are commonly used to treat rheumatoid arthritis (RA). However, NB‑DMARD can have adverse events or inadequate response that lead to premature discontinuation. Up to 50% of patients initiating MTX may discontinue at 2 years.1-3 The objective of this exploratory analysis was to evaluate real world treatment patterns and impact on healthcare resource use (HCRU) among patients whose first DMARD regimen was select NB-DMARD as monotherapy or in combination with other NB-DMARD.
Methods: This retrospective cohort study evaluated patients aged ≥18 years at index with ≥3 physician visits ≥30 days apart for RA (ICD-9 code: 714.xx) who were newly prescribed/administered a select NB-DMARD (MTX, leflunomide, sulfasalazine, hydroxychloroquine) from 2007-2011 in a de-identified electronic health record (EHR) database (Humedica). The index date was the date of the first select NB-DMARD prescription/administration in the EHR (ie, no DMARD in ≥6 months pre-index), and patients were followed for ≥1 year. Patients were categorized by initial treatment with NB-DMARD monotherapy (NBmono) or ≥2 select NB-DMARD at index (NBcombo). Patient baseline characteristics, 1-year post-index treatment patterns and HCRU were evaluated in EHR. RA-related costs were evaluated for a subset of patients with clinical information linked to healthcare claims (Optum) of commercial and Medicare Advantage health plans.
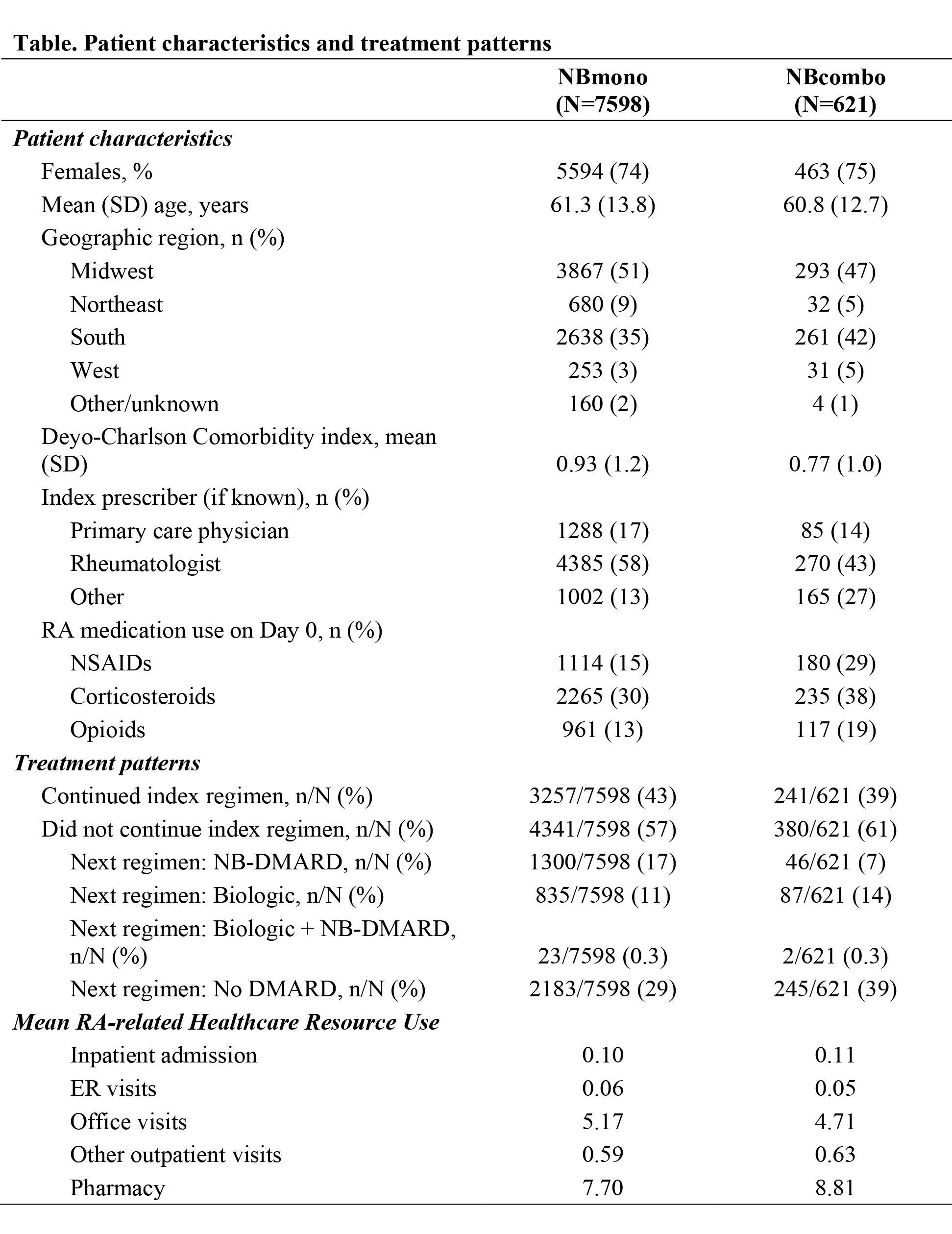
Results: Of 10,338 RA patients receiving any DMARD therapy, 73% received NBmono and 6% NBcombo; patient characteristics are presented in the Table. NBcombo patients tended to have a lower DCCI score, fewer were treated by a rheumatologist, and tended to have more NSAIDS/steroids/opioids at index; <1% of patients had other NB-DMARDs within 30 days of index. During the 1-year follow-up, NBcombo patients were less likely than NBmono patients to continue their index NB-DMARD treatment regimen; treatment patterns are presented in the Table. NBcombo patients had fewer RA-related emergency room (mean difference -0.01) and office (-0.46) visits but more prescriptions (1.1), other outpatient visits (0.04), and inpatient admissions (0.01) in the post-index period. In 204 NB-DMARD patients with claims data, the mean monthly cost was $267 (49% attributed to prescriptions, 42% to office/outpatient visits); costs for NBcombo were 6% higher vs NBmono.
Conclusion: Among RA patients treated with DMARDs, 79% received NB-DMARD (92% initiated as monotherapy). Approximately one third of NB-DMARD pts did not continue with any DMARD therapy in the 1-year follow up. NBcombo pts were less likely to continue their index treatment regimen, and only 14% initiated a biologic DMARD.
1. Lie E et al. Rheumatology 2012; 51: 670-678.
2. Lie E et al. Ann Rheum Dis 2010; 69: 671-676.
3. Bernatsky S et al. Drugs and Aging 2008; 25: 879-994.
Disclosure:
D. Wiederkehr,
Pfizer Inc,
1,
Pfizer Inc,
3;
J. Harnett,
Pfizer Inc,
1,
Pfizer Inc,
3;
R. Gerber,
Pfizer Inc,
1,
Pfizer Inc,
3;
D. Gruben,
Pfizer Inc,
1,
Pfizer Inc,
3;
E. Y. Mahgoub,
Pfizer Inc,
1,
Pfizer Inc,
3;
G. Wallenstein,
Pfizer Inc,
1,
Pfizer Inc,
3;
A. Koenig,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-real-world-experience-with-non-biologic-dmard-in-the-treatment-of-ra-data-from-an-electronic-health-record-database/