Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Abatacept is recommended as first-line biologic therapy in adult patients with moderate to severe RA. We aimed to assess real-world 1-year treatment persistence in early-line abatacept versus TNFi treated RA patients who were ACPA+ and RF+, which predict poorer functional and radiographic outcomes.
Methods: As part of a multicenter retrospective medical record review of adult RA patients with poor prognostic factors, patients were treated with abatacept or TNFi as the first biologic treatment at participating clinics (defined as early line) and had ACPA positivity, RF positivity, increased CRP, elevated ESR, or joint erosions. This analysis only included ACPA+ and/or RF+ patients. Patients in the TNFi group were treated with adalimumab, etanercept, infliximab (and biosimilars), certolizumab pegol, or golimumab. Data were collected ≥1 year from treatment initiation (8/9/11-11/14/16). Patients with Crohn’s disease, ankylosing spondylitis, ulcerative colitis, psoriatic arthritis, or anal fistula were excluded. Demographic, disease, and treatment data (start, stop, reason for discontinuation) were abstracted. Treatment persistence (continuation of index treatment with gap ≤60 days) at 1 year and time to discontinuation were reported. Multivariate logistic and Cox modeling with forward selection compared abatacept and TNFi 1-year persistence, risk of overall discontinuation, and discontinuation for disease progression, controlling for demographic and clinical characteristics (age, sex, Charlson comorbidity index (CCI), RA duration), baseline utilization, and clinic. Analyses will be repeated when >300 patients are recruited.
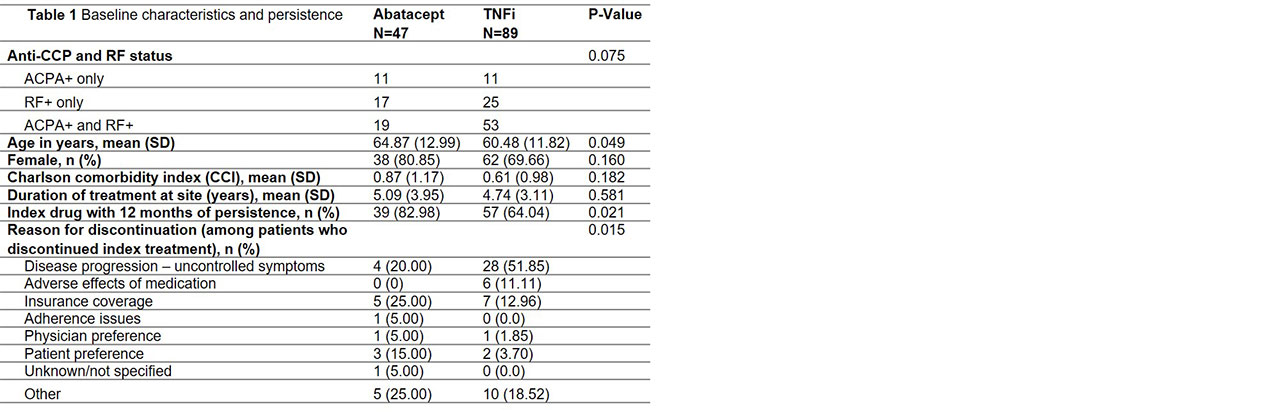
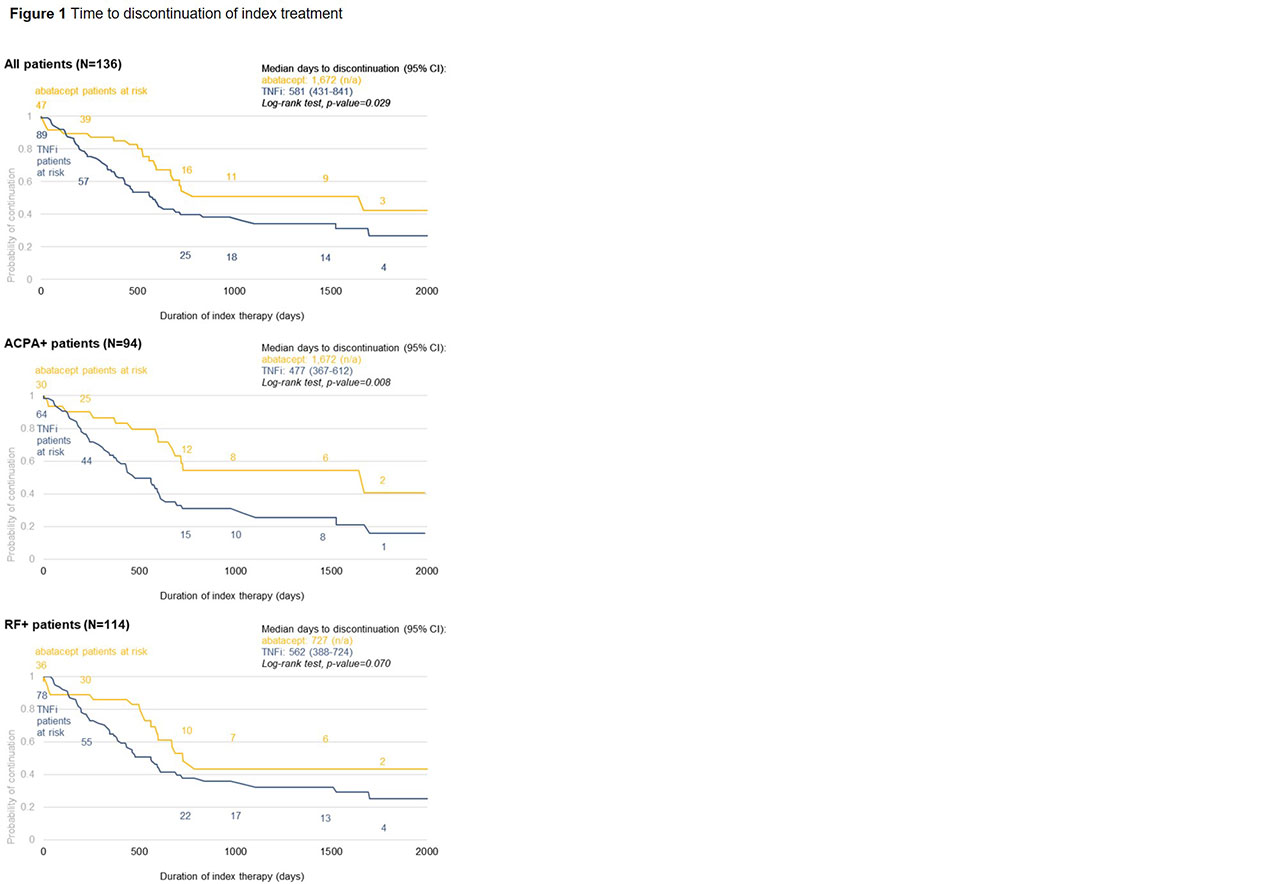
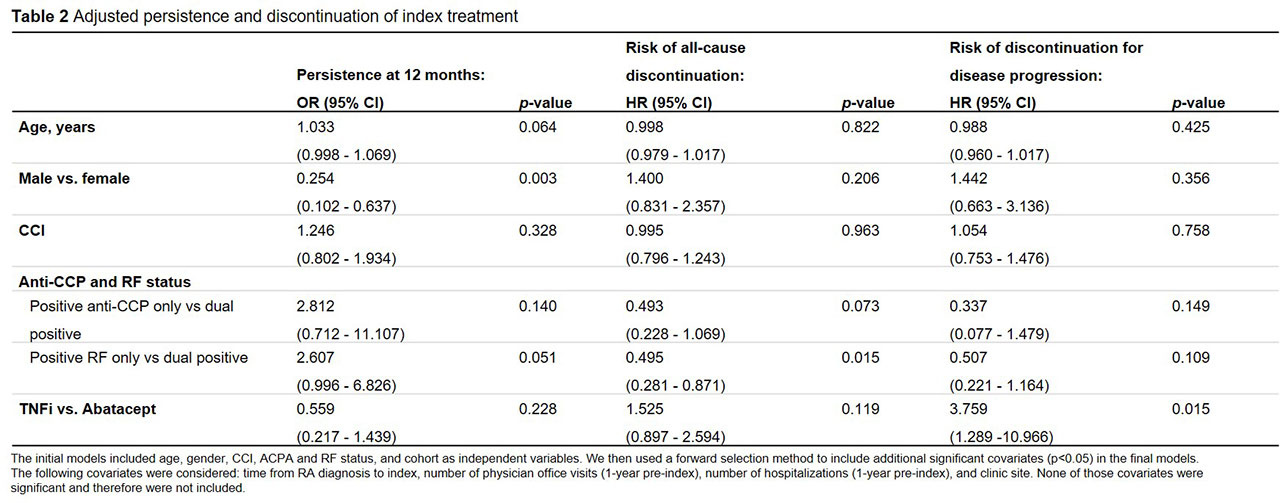
Results: Data on 136 patients (47 abatacept, 89 TNFi) were available at the time of analysis (Table 1). Abatacept patients were older than TNFi patients. There were no significant differences in gender, CCI, or duration of treatment at the clinic. Risk of discontinuation was lower in abatacept vs. TNFi overall (p=0.029) and for both ACPA+ (p=0.008) and RF+ (p=0.070) patients. Median time to discontinuation for ACPA+ and RF+ patients was 1,672 and 727 days for abatacept vs. 477 and 562 days for TNFi, respectively (Figure 1). At 1 year, 83% of abatacept vs. 64% of TNFi patients were persistent (p=0.021) (Table 1). Adjusted risk of discontinuation was higher in TNFi patients, although not statistically significant (Table 2). Odds of 1-year persistence was lower in TNFi than abatacept patients, but not statistically significant (Table 2). TNFi patients were at significantly higher risk of discontinuing index treatment due to disease progression.
Conclusion: In this analysis of ACPA+ and RF+ RA patients, unadjusted analyses demonstrate these patients are significantly more likely to be persistent to abatacept than TNFi at 1 year in a real-world setting. Abatacept patients also experienced a longer time to discontinuation than the TNFi cohort, which may be explained by the significantly lower proportion of patients discontinuing abatacept due to disease progression. Perhaps due to limited sample size, the adjusted comparison of abatacept and TNFi persistence is not significant, however, numeric trends are consistent.
To cite this abstract in AMA style:
Paul D, Han X, Yermilov I, Gibbs S, Broder M. Evaluation of Real-World Early-Line Abatacept versus Tumor Necrosis Factor Inhibitors Persistence in Rheumatoid Arthritis Patients with Anti-Citrullinated Protein Antibody or Rheumatoid Factor Positivity [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-real-world-early-line-abatacept-versus-tumor-necrosis-factor-inhibitors-persistence-in-rheumatoid-arthritis-patients-with-anti-citrullinated-protein-antibody-or-rheumatoid-factor-positiv/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-real-world-early-line-abatacept-versus-tumor-necrosis-factor-inhibitors-persistence-in-rheumatoid-arthritis-patients-with-anti-citrullinated-protein-antibody-or-rheumatoid-factor-positiv/